Advertisements
Advertisements
प्रश्न
ln which pair highest oxidation states of transition metals are found:
पर्याय
nitriles and chlorides
fluorides and chlorides
fluorides and oxides
nitriles and oxides
उत्तर
fluorides and oxides
APPEARS IN
संबंधित प्रश्न
Calculate magnetic moment of `Fe_((aq))^(2+) ion (Z=26).`
Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
What are the characteristics of the transition elements and why are they called transition elements?
Which of the d-block elements may not be regarded as the transition elements?
Compare the stability of +2 oxidation state for the elements of the first transition series.
How would you account for the following:
Cobalt (II) is stable in aqueous solutions, but in the presence of complexing reagents, it is easily oxidised.
NF3 is possible, but NF5 is not. Why?
Write balanced chemical equations for the conversion of `CrO_4^(2-)` to `Cr_2O_7^(2-)` in acidic medium and `Cr_2O_7^(2-)` to `CrO_4^(2-)`
in basic medium.
Account for the following :
Ti4+ is colourless whereas V4+ is coloured in an aqueous solutions.
Explain why transition elements form alloys.
Interstitial compounds are formed when small atoms are trapped inside the crystal lattice of metals. Which of the following is not the characteristic property of interstitial compounds?
Which of the following statements is not correct?
EΘ of Cu is + 0.34V while that of Zn is – 0.76V. Explain.
The halides of transition elements become more covalent with increasing oxidation state of the metal. Why?
Read the passage given below and answer the following question.
|
Are there nuclear reactions going on in our bodies? There are nuclear reactions constantly occurring in our bodies, but there are very few of them compared to the chemical reactions, and they do not affect our bodies much. All of the physical processes that take place to keep a human body running are chemical processes. Nuclear reactions can lead to chemical damage, which the body may notice and try to fix. The nuclear reaction occurring in our bodies is radioactive decay. This is the change of a less stable nucleus to a more stable nucleus. Every atom has either a stable nucleus or an unstable nucleus, depending on how big it is and on the ratio of protons to neutrons. The ratio of neutrons to protons in a stable nucleus is thus around 1 : 1 for small nuclei (Z < 20). Nuclei with too many neutrons, too few neutrons, or that are simply too big are unstable. They eventually transform to a stable form through radioactive decay. Wherever there are atoms with unstable nuclei (radioactive atoms), there are nuclear reactions occurring naturally. The interesting thing is that there are small amounts of radioactive atoms everywhere: in your chair, in the ground, in the food you eat, and yes, in your body. The most common natural radioactive isotopes in humans are carbon-14 and potassium-40. Chemically, these isotopes behave exactly like stable carbon and potassium. For this reason, the body uses carbon-14 and potassium-40 just like it does normal carbon and potassium; building them into the different parts of the cells, without knowing that they are radioactive. In time, carbon-14 atoms decay to stable nitrogen atoms and potassium-40 atoms decay to stable calcium atoms. Chemicals in the body that relied on having a carbon-14 atom or potassium-40 atom in a certain spot will suddenly have a nitrogen or calcium atom. Such a change damages the chemical. Normally, such changes are so rare, that the body can repair the damage or filter away the damaged chemicals. The natural occurrence of carbon-14 decay in the body is the core principle behind carbon dating. As long as a person is alive and still eating, every carbon-14 atom that decays into a nitrogen atom is replaced on average with a new carbon-14 atom. But once a person dies, he stops replacing the decaying carbon-14 atoms. Slowly the carbon-14 atoms decay to nitrogen without being replaced, so that there is less and less carbon-14 in a dead body. The rate at which carbon-14 decays is constant and follows first order kinetics. It has a half-life of nearly 6000 years, so by measuring the relative amount of carbon-14 in a bone, archeologists can calculate when the person died. All living organisms consume carbon, so carbon dating can be used to date any living organism, and any object made from a living organism. Bones, wood, leather, and even paper can be accurately dated, as long as they first existed within the last 60,000 years. This is all because of the fact that nuclear reactions naturally occur in living organisms. |
Researchers have uncovered the youngest known dinosaur bone, dating around 65 million years ago. How was the age of this fossil estimated?
The orientation of an atomic orbital is governed by
The spin magnetic moment of cobalt in the compound Hg [Co(SCN)4] is:-
Passing H2S gas into a mixture of Mn2+ and Ni2+, Cu2+, ions in an acidified aqueous solution precipitates.
Match List - I with List - II.
| List - I | List - II | ||
| (a) | \[\ce{[Fe(CN)6]^3-}\] | (i) | 5.92 BM |
| (b) | \[\ce{[Fe(H2O)6]^3+}\] | (ii) | 0 BM |
| (c) | \[\ce{[Fe(CN)6]^4-}\] | (iii) | 4.90 BM |
| (d) | \[\ce{[Fe(H2O)6]^2+}\] | (iv) | 1.73 BM |
Choose the correct answer from the options given below.
Why is the `"E"_(("V"^(3+)//"V"^(2+)))^"o"` value for vanadium comparatively low?
Account for the following:
Transition metals form alloys.
The disproportionation of \[\ce{MnO^{2-}_4}\] in acidic medium resulted in the formation of two manganese compounds A and B. If the oxidation state of Mn in B is smaller than that of A, then the spin-only magnetic moment (µ) value of B in BM is ______. (Nearest integer)
Account for the following:
Ce4+ is a strong oxidising agent.
Which one among the following metals of the 3d series has the lowest melting point?
Which of the following transition metals shows +1 and +2 oxidation states?
The trend of which property is represented by the following graph?
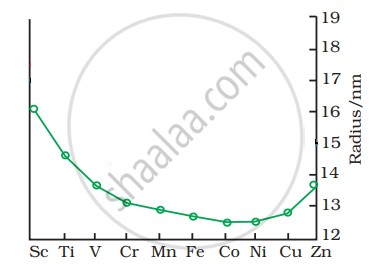
Give two similarities in the properties of Sc and Zn.
Write the ionic equation for reaction of KI with acidified KMnO4.
Give a reason for the following.
Some transition metals and their compounds get attracted towards the magnetic field.
