Advertisements
Advertisements
प्रश्न
Name the gas produced when chlorine water is exposed to sunlight.
उत्तर
Oxygen gas
APPEARS IN
संबंधित प्रश्न
Certain blank spaces are left in the following table and these are labelled as A, B, C, D
and E. Identify each of them
| Lab preparation of | Reactants used | Products formed | Drying Agent | Method of collection |
|
| 1 | HCl gas | NaCl + H2SO4 |  |
conc. H2SO4 | 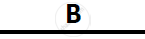 |
| 2 | NH3 gas |
|
Mg(OH)2 NH3 |
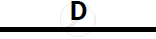 |
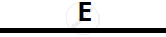 |
Write a balanced chemical equation for the action of hydrochloric acid on sodium bicarbonate.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
Name the drying agent used and justify your choice.
The following are pertaining to the laboratory preparation of hydrogen chloride gas.
State a safety precaution you would take during the preparation of hydrochloric acid.
For the preparation of hydrochloric acid in the laboratory:
Why is the direct absorption of hydrogen chloride gas in water not feasible?
Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following.
- Name the acid used. Why is this particular acid preferred to other acids?
- Give the balanced equation for the reaction.
- Name the drying agent used in drying hydrogen chloride gas.
- Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas. Why?
- Why is the direct absorption of \[\ce{HCl}\] gas in water not feasible?
- What arrangement is done to dissolve \[\ce{HCl}\] gas in the water?
Name the drying agents phosphorus pentoxide and calcium oxide are good drying agent but they cannot be used to dry hydrogen chloride gas. Why?
How will you prove that the gas prepared is HCI?
Explain, why (or give reasons for)
HCI gas does not conduct electricity, but hydrochloric acid conducts electricity.
What is aqua-regia?
Mention the reaction condition and give balanced equation to obtain: Cl2 gas from HCI gas.
Choose the correct answer from the options given below:
Bleaching powder reacts with few drops of concentrated HCl to give
Write the equation for the reaction which takes place in question(a).
Hydrogen chloride gas, being highly soluble in water, is dried by ______.
One chemical test that would enable you to distinguish between the following pair of chemicals. Describe what happens with each chemical or state 'no visible reaction'.
Sodium sulphate solution and sodium chloride solution.
Answer the following question related to the laboratory preparation of the hydrogen chloride gas:
State the temperature required in the preparation.
State a relevant reason for the following:
Hydrogen chloride gas cannot be dried over quick lime.
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
Complete and balance the following reaction, state whether dilute or conc. acid is used.
\[\ce{NH4OH + HCl ->}\]
