Advertisements
Advertisements
प्रश्न
What is a cyclic process?
उत्तर
This is a thermodynamic process in which the thermodynamic system returns to its initial state after undergoing a series of changes. Since the system comes back to the initial state, the change in the internal energy is zero. In the cyclic process, heat can flow into the system and heat flows out of the system. From the first law of thermodynamics, the net heat transferred to the system is equal to the work done by the gas.
Qnet = Qin – Qout = W (for a Cyclic Process)
APPEARS IN
संबंधित प्रश्न
Heating a gas in a constant volume container is an example of which process?
Draw a p-V diagram showing positive work with varying pressure.
Explain graphically (i) positive work with varying pressure, (ii) negative work with varying pressure, and (iii) positive work at constant pressure.
Write a note on free expansion.
Derive the work done in an isothermal process.
What are the limitations of the first law of thermodynamics?
In a petrol engine, (internal combustion engine) air at atmospheric pressure and temperature of 20°C is compressed in the cylinder by the piston to `1/8` of its original volume. Calculate the temperature of the compressed air. (For air γ = 1.4)
In which of the following processes, beat is neither absorbed nor released by a system?
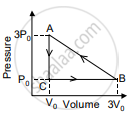
In the figure shown here, the work done in the process ACBA is ______.
When an inflated ballon is suddenly burst, why is the emerging air slightly cooled?
