Advertisements
Advertisements
प्रश्न
What is a cyclic process?
उत्तर
This is a thermodynamic process in which the thermodynamic system returns to its initial state after undergoing a series of changes. Since the system comes back to the initial state, the change in the internal energy is zero. In the cyclic process, heat can flow into the system and heat flows out of the system. From the first law of thermodynamics, the net heat transferred to the system is equal to the work done by the gas.
Qnet = Qin – Qout = W (for a Cyclic Process)
APPEARS IN
संबंधित प्रश्न
A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in Figure

Its volume is then reduced to the original value from E to F by an isobaric process. Calculate the total work done by the gas from D to E to F
Draw a p-V diagram of the reversible process.
Draw a p-V diagram showing positive work with varying pressure.
Draw a p-V diagram showing positive work at constant pressure.
Explain the thermodynamics of the isobaric process.
Give an expression for work done in an isothermal process.
Draw the PV diagram for the adiabatic process.
Explain in detail the isochoric process.
Consider P-V diagram for an ideal gas shown in figure.
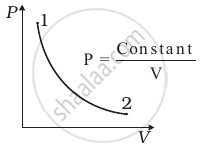
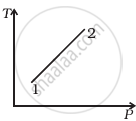
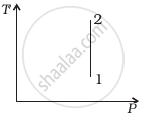
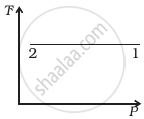
Out of the following diagrams (figure), which represents the T-P diagram?
 (i) |
 (ii) |
 (iii) |
 (iv) |
In a cyclic process, if ΔU = internal energy, W = work done, Q = Heat supplied then ______.
