Advertisements
Advertisements
प्रश्न
What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is passed through this solution?
उत्तर
When aqueous KCN solution is added to aqueous copper sulphate solution, [Cu(CN)4]2− coordination species is obtained as follows:
\[\ce{[Cu(H2O)4]^2+ + 4CN- -> [Cu(CN)4]^2- + 4H2O}\]
The coordination species thus formed [Cu(CN)4]2− is very stable because CN– is a strong ligand. Hence, on passing H2S gas through this solution, CuS precipitate is not obtained because free Cu2+ ions are unavailable.
APPEARS IN
संबंधित प्रश्न
What is meant by unidentate ligand?
Write IUPAC name of the following Complex [Cr(NH3)3Cl3]
Write the structures of compounds A, B and C in the following reactions
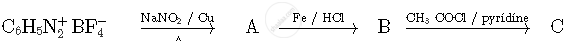
Write the IUPAC names of the following coordination compounds:
[Cr(NH3)4(H2O)2]Cl3
The oxidation number of Fe in K4[Fe(CN)6] is ____________.
Which of the following complexes formed by \[\ce{Cu^2+}\] ions is most stable?
The correct \[\ce{IUPAC}\] name of \[\ce{[Pt(NH3)2Cl2]}\] is ______.
Which of the following complexes are heteroleptic?
(i) \[\ce{[Cr(NH3)6]^{3+}}\]
(ii) \[\ce{[Fe(NH3)4]Cl2]^+}\]
(iii) \[\ce{[Mn(CN)6]^{4-}}\]
(iv) \[\ce{[Co(NH3)4]Cl2]}\]
A coordination compound \[\ce{CrCl3.4H2O}\] precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write structural formula of the compound and name it.
A complex of the type \[\ce{[M(AA)2X2]^{n+}}\] is known to be optically active. What does this indicate about the structure of the complex? Give one example of such complex.
Ethylene diaminetetraacetate (EDTA) ion is ______
What are Heteroleptic complexes?
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
What is meant by the chelate effect? Give an example.
Explain the following, giving two examples:
Coordination polyhedron
Give two examples of unidentate ligand.
