Advertisements
Advertisements
प्रश्न
Write the reaction of aromatic primary amine with nitrous acid.
उत्तर
Aromatic amine react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at 273 − 278 K to form stable aromatic diazonium salts, i.e., NaCl and H2O.
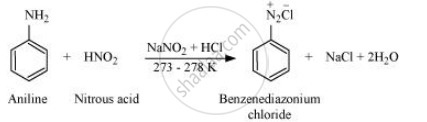
APPEARS IN
संबंधित प्रश्न
How do you convert the following: Ethanenitrile to ethanamine
Give the structure of A, B and C in the following reaction:
\[\ce{C6H5N2Cl ->[CuCN] A ->[H2O/H+] B ->[NH3][\Delta] C}\]
Explain the mechanism of action of hydroiodic acid on 3-methylbutan-2-ol.
Give the structures of A, B and C in the following reactions :

Answer the following
Explain Gabriel phthalimide synthesis.
Mendius reaction is used to convert _____________
Write reactions to bring about the following conversions.
Acetamide to Ethylamine
Explain Hoffmann’s exhaustive alkylation with suitable reactions.
Why cannot aniline be prepared by Gabriel phthalimide synthesis?
What is the molar mass of the amine formed when acetamide undergoes Hofmann bromamide degradation?
The reduction of alkyl cyanide with sodium and ethanol to give primary amines is, ____________.
Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one \[\ce{CH2}\] group in the carbon chain, the reagent used as source of nitrogen is ______.
Amongst the given set of reactants, the most appropriate for preparing 2° amine is ______.
The best reagent for converting 2–phenylpropanamide into 2-phenylpropanamine is ______.
Identify A and B in the following reaction.
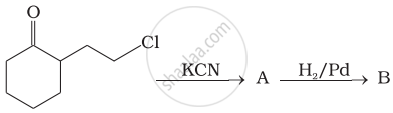
Which of the following amines can be prepared by Gabriel phthalimide reaction?
Amides can be converted into amines by the reaction named ______.
Identify the compo ds A and B in the following reactions:
\[\ce{A ->[Nitrating mixture] B ->[(i) Sn/cone. HCI][(ii) NaOH] Aniline}\]
Write a short note on the following:
Ammonolysis
