Advertisements
Advertisements
Question
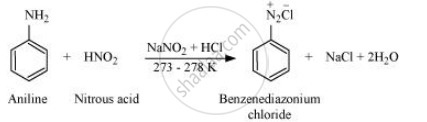
Write the reaction of aromatic primary amine with nitrous acid.
Solution
Aromatic amine react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at 273 − 278 K to form stable aromatic diazonium salts, i.e., NaCl and H2O.
APPEARS IN
RELATED QUESTIONS
How are propan-1-amine and propan-2-amine prepared from oxime?
How do you convert the following: Ethanenitrile to ethanamine
Give the structures of A, B and C in the following reactions :
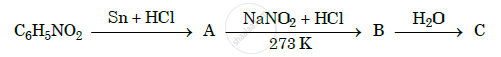
Answer the following
Explain the ammonolysis of alkyl halides.
Identify compound 'B' in following series of reactions?
\[\ce{Acetonitrile ->[Na/alcohol] A ->[NaNO2/dil.HCI] B}\]
Alkyl cyanides on reduction by sodium and ethanol give primary amines. This reaction is called as ____________.
Identify the INCORRECT statement regarding Hofmann bromamide reaction.
In aqueous phase the order of basic strength of alkylamine is ______.
Which of the following methods of preparation of amines will give same number of carbon atoms in the chain of amines as in the reactant?
Suggest a route by which the following conversion can be accomplished.

How will you carry out the following conversions?
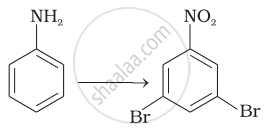
How will you carry out the following conversions?
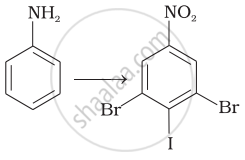
Assertion: Aromatic 1° amines can be prepared by Gabriel Phthalimide Synthesis.
Reason: Aryl halides undergo nucleophilic substitution with anion formed by phthalimide.
Describe Gabriel's phthalimide synthesis. (Give reaction)
Which of the following reaction DOES NOT involve Hoffmann bromamide degradation?
Which of the following statement(s) is/are incorrect in case of Hofmann bromamide degradation?
Write a short note on the following:
Ammonolysis
Write a short note on the following:
Ammonolysis.
Write short notes on the following:
Ammonolysis
