HSC Science (General)
HSC Science (Electronics)
HSC Science (Computer Science)
Academic Year: 2023-2024
Date & Time: 23rd July 2024, 11:00 am
Duration: 3h
Advertisements
General instructions:
The question paper is divided into four sections:
- Section A: Q. No. 1 contains Ten multiple-choice type of questions carrying One mark each. Q. No. 2 contains Eight very short answer type of questions carrying One mark each.
- Section B: Q. No. 3 to Q. No. 14 contain twelve short answer type of questions carrying two marks each. (Attempt any Eight).
- Section C: Q. No. 15 to Q. No. 26 contains Twelve short answer type of questions carrying Three marks each. (Attempt any Eight).
- Section D: Q. No. 27 to Q. No. 31 contain Five long answer type of questions carrying Four marks each. (Attempt any Three).
- Use of the log table is allowed. Use of calculator is not allowed.
- Figures to the right indicate full marks.
- For each multiple choice type of question, it is mandatory to write the correct answer along with its alphabet. e.g. (a)........./ (b)........./ (c)........./ (d) etc.
Only the first attempt will be considered for evaluation. - Physical Constants:
Given: R = 8.314 J K−1 mol−1
NA = 6.022 × 1023
F = 96500C
Integrated rate law equation for a zero order reaction is ______.
`K_t = [A]_0 - [A]_t`
`K_t = 2.303 log_10 [A]_0/[A]_t`
`K = ([A]_t - [A]_0)/t`
`K_t = 2.303 log_10 ([A]_t)/[A]_0`
Chapter:
The spin-only magnetic moment of Fe+2 ion is ______.
3.806 BM
4.899 BM
5.796 BM
6.817 BM
Chapter:
The relation between radius of sphere and edge length in simple cubic lattice is ______.
`sqrt3r = 4a`
`sqrt3a = 4r`
`r = a/2`
`sqrt2a = 4r`
Chapter:
The monomer used in the preparation of thermocol is ______.
vinyl chloride
acrylamide
butadiene and acrylonitrile
styrene
Chapter:
Which of the following α-amino acids do not contain chiral centre?
Alanine
Valine
Leucine
Glycine
Chapter:
Neutral complex in the following is ______.
\[\ce{[Co(NO2)3(NH3)3]}\]
\[\ce{[Co(NH3)5CO3]Cl}\]
\[\ce{Na3[Co(NO2)6]}\]
\[\ce{[Co(C2O4)3]^3-}\]
Chapter:
The reaction used to convert toluene to benzaldehyde is known as ______.
Etard's reaction
Gatterman-Koch formylation reaction
Friedel-Crafts reaction
Swartz reaction
Chapter:
A better reagent to convert primary alcohol to aldehyde is ______.
Chromic anhydride
Pyridinium chlorochromate
Potassium permanganate
Potassium dichromate
Chapter:
Write the name of insecticide which is effectively used instead of DDT.
Chapter:
Write the name or formula of hydrocarbon formed when methyl magnesium iodide is treated with ammonia.
Chapter:
Calculate emf of the cell at 25°C.
Zn(s) | Zn2+ (0.08 M) || Cr3+ (0.1 M) | Cr(s)
E°Zn = −076V, E°Cr = −0.74V.
Chapter: [0.05] Electrochemistry
Write the electronic configuration of Zn2+ ion. (Atomic number of zinc is 30).
Chapter:
Write the IUPAC name of amine formed when acetamide undergoes Hofmann bromamide degradation.
Chapter:
Write the condition for spontaneity of reaction with respect to entropy.
Chapter:
Write the relation between van't Hoff factor and degree of dissociation of an electrolyte.
Chapter:
The normal boiling point of ethyl acetate is 77.06 °C. A solution of 50 g of non-volatile solute in 150 g ethyl acetate boils at 82.60 °C. Calculate molar mass of solute. [Kb = 2.77°C kg mol−1]
Chapter:
Write the mathematical equation for the first law of thermodynamics for:
Isothermal process
Chapter: [0.04] Chemical Thermodynamics
Write mathematical equation of first law of thermodynamics for Isochoric process.
Chapter: [0.03] Chemical Thermodynamics and Energetic
Complete the following equation:
XeF2 + H2O →
Chapter: [0.07] Elements of Groups 16, 17 and 18 [7.04] Group 18 Elements
Complete the following equation:
\[\ce{Xe + F_2 ->[Electric discharge][Low temperature]?}\]
Chapter:
Differentiate between ionic crystals and covalent crystals.
Chapter:
What is the mass of Cu metal produced at the cathode during the passage of 5 ampere of current through CuSO4 solution for 100 minutes? [Molar mass of Cu = 63.5 g mol−1].
Chapter:
Define buffer solution.
Chapter: [0.03] Ionic Equilibria
Advertisements
Write the Henderson Hasselbalch equation for acidic buffer.
Chapter:
Explain any three principles of green chemistry.
Chapter: [0.16] Green Chemistry and Nanochemistry
Write the reaction for the preparation of Nylon-6.
Chapter: [0.15] Polymers
Define pyrometallurgy.
Chapter: [0.08] Transition and Inner Transition Elements
Write the reaction when ethylamine is treated with excess of ethyl bromide.
Chapter:
Write the reaction when ethylamine is treated with Hinsberg's reagent.
Chapter:
Construct a cell using standard hydrogen electrode and zinc electrode. Write its cell reaction and cell representation. Calculate cell potential of a cell with 0.01 M Zn2+ ions. Standard potential of a cell is + 0.76 V.
Chapter:
State Henry's law.
Chapter: [0.02] Solutions and Colligative Properties
Calculate the standard enthalpy of the reaction:
SiO2(s) + 3C(graphite) → SiC(s) + 2CO(g) from the following reactions:
- Si(s) + O2(g) → SiO2(s), ΔrH° = −911kJ
- 2C(graphite) + O2(g) → 2CO(g), ΔrH° = −221kJ
- Si(s) + C(graphite) → SiC(s), ΔrH° = −65.3kJ
Chapter: [0.04] Chemical Thermodynamics
Derive Ostwald's dilution law for the CH3COOH.
Chapter: [0.03] Ionic Equilibria
Obtain relation between solubility product and its solubility for Al(OH)3
Chapter:
In a first-order reaction A → B, 60% of a given sample of a compound decomposes in 45 mins. What is the half-life of reaction? Also, write the rate law equation for the above first-order reaction.
Chapter: [0.06] Chemical Kinetics
Write the factors which are related to the colour of transition metal ions.
Chapter: [8.01] D-block Elements
Write the alloy used in fischer Tropsch process.
Chapter:
Advertisements
Write anomalous behaviour of oxygen with respect to atomicity.
Chapter:
Write anomalous behaviour of oxygen with respect to magnetic property.
Chapter:
Write anomalous behaviour of oxygen with respect to the oxidation state.
Chapter:
Draw the zwitter ion structure for sulphanilic acid.
Chapter:
Identify A, B, C and rewrite the chemical reactions:
\[\ce{H3C - CH2Cl + KOH_{(aq)} ->[Δ] 'A' ->[hot Cu][powder] 'B' ->[K2Cr2O7][dil H2SO4] 'C'}\]
Chapter:
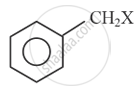
Explain optical isomerism in 2-chlorobutane.
Chapter: [0.1] Halogen Derivatives
Write Clemmenson's reduction reaction of propanone.
Chapter:
How is dioxygen prepared in the laboratory from KClO3?
Chapter: [0.07] Elements of Groups 16, 17 and 18
How is phenol converted into the following?
benzoquinone
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
Write the chemical reaction to convert phenol to cyclohexanol.
Chapter:
Define the Standard enthalpy of combustion.
Chapter: [0.04] Chemical Thermodynamics
Define the Enthalpy of atomization
Chapter: [0.03] Chemical Thermodynamics and Energetic
Write the structure of D-ribose.
Chapter: [0.14] Biomolecules
Distinguish between Order and Molecularity of reaction.
Chapter: [0.06] Chemical Kinetics
Answer the following in brief.
Write electrode reactions for the electrolysis of aqueous NaCl.
Chapter: [0.05] Electrochemistry
Gold crystallises into face-centred cubic cells. The edge length of a unit cell is 4.08 × 10–8 cm. Calculate the density of gold. [Molar mass of gold = 197 g mol–1]
Chapter: [0.01] Solid State
Answer the following
Which flower is an example of self-cleaning?
Chapter: [0.16] Green Chemistry and Nanochemistry
Write the monomer used for the preparation of PVC.
Chapter:
Explain the formation of [Co(NH3)6]3+ ion on the basis of Valence bond theory.
Chapter:
Give a balanced equation for the following:
Conversion of acetic acid to ethyl acetate.
Chapter:
Write chemical reactions for the following conversion:
Acetic acid into ethyl alcohol
Chapter: [0.11] Alcohols, Phenols and Ethers [11.01] Alcohols [11.02] Phenols [11.03] Ethers
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 12th Standard Board Exam Chemistry with solutions 2023 - 2024
Previous year Question paper for Maharashtra State Board 12th Standard Board Exam Chemistry-2024 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 12th Standard Board Exam.
How Maharashtra State Board 12th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.