Advertisements
Advertisements
Question
A person of mass 60 kg wants to lose 5kg by going up and down a 10 m high stairs. Assume he burns twice as much fat while going up than coming down. If 1 kg of fat is burnt on expending 7000 kilo calories, how many times must he go up and down to reduce his weight by 5 kg?
Solution
Given, the height of the stair = h = 10 m
The energy produced by burning 1 kg of fat = 7000 cal
∴ The energy produced by burning 5 kg of fat = 5 × 7000 = 35000 kcal
35 × 106 cal
The energy utilised in going up and down one time
= `mgh + 1/2 mgh`
= `3/2 mgh`
= `3/2 xx 60 xx 10 xx 10`
= 9000 J
= `9000/4.2`
= `3000/1.4` cal
∴ A number of times, the person has to go up and down the stairs
= `(35 xx 10^6)/(3000/1.4)`
= `(35 xx 1.4 xx 10^6)/3000`
= 16.3 × 103 times
APPEARS IN
RELATED QUESTIONS
Explain why Air pressure in a car tyre increases during driving.
A cylinder containing a gas is lifted from the first floor to the second floor. What is the amount of work done on the gas? What is the amount of work done by the gas? Is the internal energy of the gas increased? Is the temperature of the gas increased?
The outer surface of a cylinder containing a gas is rubbed vigorously by a polishing machine. The cylinder and its gas become warm. Is the energy transferred to the gas heat or work?
When we rub our hands they become warm. Have we supplied heat to the hands?
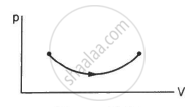
Consider the process on a system shown in figure. During the process, the work done by the system ______________ .
Consider the following two statements.
(A) If heat is added to a system, its temperature must increase.
(B) If positive work is done by a system in a thermodynamic process, its volume must increase.
A gas is contained in a metallic cylinder fitted with a piston. The piston is suddenly moved in to compress the gas and is maintained at this position. As time passes the pressure of the gas in the cylinder ______________ .
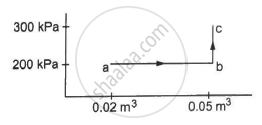
A substance is taken through the process abc as shown in figure. If the internal energy of the substance increases by 5000 J and a heat of 2625 cal is given to the system, calculate the value of J.
In insulated systems, the amount of external work done by the gas is proportional to:
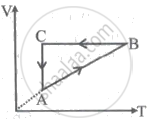
A cyclic process ABCA is shown in the V-T diagram. A process on the P-V diagram is ______.