Advertisements
Advertisements
Questions
Account for the following:
Although the amino group is o, p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
Give reasons for the following:
Although the –NH2 group is o/p directing in an electrophilic substitution reaction, yet aniline on nitration gives a good yield of m-nitroaniline.
Solution 1
Nitration is carried out in an acidic medium. In an acidic medium, aniline is protonated to give anilinium ions (which are meta-directing).
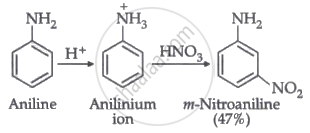
For this reason, aniline on nitration gives a substantial amount of m-nitroaniline.
Solution 2
Nitration is usually carried out with a mixture of conc. HNO3 + conc. H2SO4. In the presence of these acids, most of the aniline gets protonated to form an anilinium ion. Therefore, in the presence of acids, the reaction mixture consists of aniline and anilinium ions. Now, the –NH2 group in aniline is activating and p-directing, while the \[\ce{-NH+3}\] group in the anilinium ion is deactivating and m-directing. Nitration of aniline (due to steric hindrance at the o-position) mainly gives p-nitroaniline; the nitration of anilinium ions gives m-nitroaniline. In actual practice, approx. a 1 : 1 mixture of p-nitroaniline and m-nitroaniline is obtained. Thus, nitration of aniline gives a substantial amount of m-nitroaniline due to protonation of the amino group.
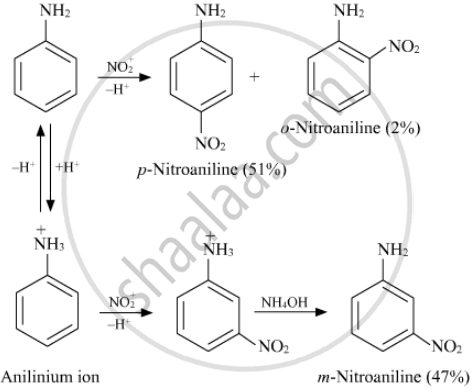
APPEARS IN
RELATED QUESTIONS
What is the action of acetic anhydride on ethylamine?
What is the action of the following reagents on aniline?
Bromine water
Give reasons for the following:
Aniline does not undergo Friedel- Crafts reaction.
Write the structures of main products when aniline reacts with the following reagents :
Br2 water
Write the structures of main products when aniline reacts with the following reagents :
(CH3CO)2O/pyridine
Write the chemical equations involved when aniline is treated with the following reagents:
Br2 water
What is the action of the following reagents on aniline?
Acetic anhydride
A solution contains 1 g mol. each of p-toluene diazonium chloride and p-nitrophenyl diazonium chloride. To this 1 g mol. of alkaline solution of phenol is added. Predict the major product. Explain your answer.
Assertion: N, N-Diethylbenzene sulphonamide is insoluble in alkali.
Reason: Sulphonyl group attached to nitrogen atom is strong electron-withdrawing group.
When bromination of aniline is carried out by protecting – NH2. The major product is
Give reasons for the following observation:
Aniline is acetylated before nitration reaction.
Give reasons for the following observation:
Aniline does not react with methyl chloride in the presence of anhydrous AlCl3 catalyst.
In the following reaction, the reason for why meta-nitro product also formed is:

How can the activating effect of the −NH2 group in aniline be controlled?
Assertion (A): Bromination of benzoic acid, gives m-bromobenzoic acid.
Reason (R): Carboxyl group increases the electron density at the meta position.
Aniline does not give Friedel-Crafts reaction. Give a reason.
