Advertisements
Advertisements
Question
An organic compound gives hydrogen on reaction with sodium metal. It forms an aldehyde with molecular formula C2H4O on oxidation with pyridinium chlorochromate. Give the chemical equations in support of these observations.
Solution
Ethyl alcohol gives hydrogen on reaction with sodium metal. On oxidation with PCC ethanol will form acetaldehyde (C2H4O).
\[\ce{\underset{\text{Ethyl alcohol}}{2CH3 - CH2 - OH} ->[2Na] \underset{\text{Sodium ethoxide}}{2CH3 - CH2 - O- Na+} + {H_{2(g)}} ^}\]
\[\ce{\underset{\text{Ethyl alcohol}}{CH3 - CH2 - OH} ->[{[O]}][PCC] \underset{\ce{Acetaldehyde(C2H4O)}}{CH3 - CHO} + H2O}\]
RELATED QUESTIONS
Answer in brief.
Explain why phenol is more acidic than ethyl alcohol.
Answer in brief.
Explain why p-nitrophenol is a stronger acid than phenol.
When vapours of tert.butyl alcohol are passed over hot copper, it gives _____________
Reaction between hot conc. HI and anisole gives ______________
Arrange the following in decreasing order of acid strength.
CH3OH, CH3–CH2–OH, CH3–CH(OH)–CH3, (CH3)3–C–OH
Write the name of major product when anisole reacts HI at 398 K
Write chemical equation of acetyl chloride with phenol
An unknown alcohol is treated with Lucas reagent. Explain how you will determine whether the alcohol is primary, secondary or tertiary. Indicate by chemical equation the reaction between isopropyl alcohol and Lucas reagent.
\[\ce{C3H8O ->[KMnO4][(Oxidation)] C3H6O2}\]
The compound C3H8O is a/an ____________.
____________ is used for silvering mirrors.
The CORRECT decreasing order of boiling points for isomeric primary (1°), secondary (2°) and tertiary (3°) alcohols is ____________.
Propane when treated with cold cone. H2SO4 forms a compound which on heating with water gives ______.
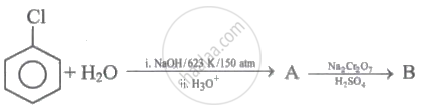
Identify 'A' and 'B' in the following series of reactions.
The product 'C' in the following reaction is:
\[\ce{CH3CH2Br ->[alc. KCN] {'A'} ->[H3O^+][\Delta] {'B'} ->[i. LiAlH4][ii. H3O^+] {'C'}}\]
Which of the following is INCORRECT regarding phenol?
Sodium benzene sulphonate reacts with NaOH and then on acidic hydrolysis, it gives __________.
In Raschig's method for synthesis of phenol, the reactants used are ____________.
(I)
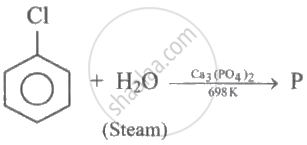
(II) \[\ce{P + Zn ->[\Delta] Q}\]
(III) \[\ce{P ->[Na2Cr2O7][H2SO4] R}\]
Q and R are respectively:
Which following reagent is used to obtain alkene from alcohol?
The number of σ bonds in carbolic acid is ______.
Identify the product obtained when phenol is treated with bromine water?
The compound which reacts fastest with Lucas reagent at room temperature is ______.
The product C in the following reaction is

The order of reactivity of hydrogen halides with ether is as follows.
What is the action of nitrous acid on aniline?
Explain the reaction of 1° and 2° alcohol with oxidising agent chromic anhydride (CrO3).
Explain: Phenols are acid while alcohol is neutral.
Convert the following :
cumene to phenol.
