Advertisements
Advertisements
Question
Can a Van der Waals gas with a = 0 be liquefied? explain.
Solution
- a = 0 for a Van der Waals gas i.e. for a real gas. Van der Waals constant a = 0. It cannot be liquefied.
- If a = 0, there is very less interaction between the molecules of gas.
- ‘a’ is the measure of the strength of Van der Waals force of attraction between the molecules of the gas.
- If a is equal to zero, the Van der Waals force of attraction is very less and the gas cannot be liquefied.
APPEARS IN
RELATED QUESTIONS
Calculate the volume occupied by 8.8 g of CO2 at 31.1°C and 1 bar pressure. R = 0.083 bar L K–1 mol–1.
Compressibility factor for CO2 at 400 K and 71.0 bar is 0.8697. The molar volume of CO2 under these conditions is
25 g of each of the following gases are taken at 27°C and 600 mm Hg pressure. Which of these will have the least volume?
In what way real gases differ from ideal gases.
Suppose there is a tiny sticky area on the wall of a container of gas. Molecules hitting this area stick there permanently. Is the pressure greater or less than on the ordinary area of walls?
A plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in Figure. Which of the following order of pressure is correct for this gas?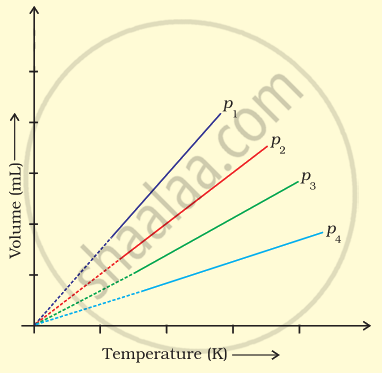
Compressibility factor, Z, of a gas is given as Z = `(pV)/(nRT)`. For real gas what will be the effect on value of Z above Boyle’s temperature?
Match the following graphs of ideal gas with their co-ordinates:
| Graphical representation | x and y co-ordinates |
(i)  |
(a) pV vs. V |
(ii) 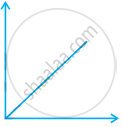 |
(b) p vs. V |
(iii) 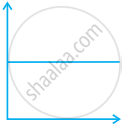 |
(c) p vs. `1/V` |
In van der Waal's equation for the real gas, the expression for the net force of attraction amongst the gas molecules is given by:
Choose the correct option for the total pressure (in atm.) in a mixture of 4g \[\ce{O2}\] and 2g \[\ce{H2}\] confined in a total volume of one litre at 0°C is ______.
[Given R = 0.082 L atm mol−1K−1, T = 273 K]
