Advertisements
Advertisements
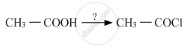
Question
Complete the synthesis by giving missing starting material, reagent or product.
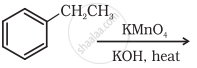
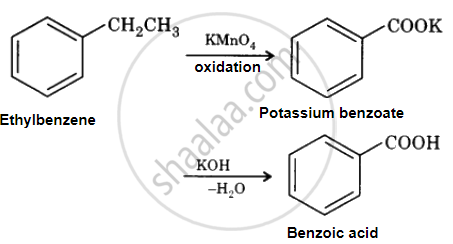
Solution
APPEARS IN
RELATED QUESTIONS
How is carbolic acid prepared from chlorobenzene?
Write the structures of A and B in the following reactions
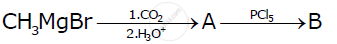
Predict the products of the following reactions:
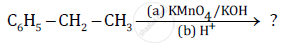
Name the reagents used in the following reactions:

Show how the following compound can be converted to benzoic acid.
Ethylbenzene
Show how the following compound can be converted to benzoic acid.
Acetophenone
Show how the following compound can be converted to benzoic acid.
Phenylethene (Styrene)
An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
m-Nitrobenzoic acid
Name the reagents used in the following reactions:
The functional group present in triacylglycerol is _______.
Through which of the following reactions number of carbon atoms can be increased in the chain?
(i) Grignard reaction
(ii) Cannizaro’s reaction
(iii) Aldol condensation
(iv) HVZ reaction
Substitution of one alkyl group by replacing hydrogen of primary amines
Which of the following substance produced acetaldehyde on dry distillation?
Benzoic acid can be obtained by the oxidation of all of the following EXCEPT ______.
The end product Y in the sequence of reaction:
\[\ce{RX ->[CN^-] X ->[NaOH] Y}\] is:
Alkaline hydrolysis of C4H8Cl2 gives a compound (A) which on heating with NaOH and I2 produces a yellow precipitate of CHI3. The compound (A) should be ______.
Hex-4-ene-2-ol on treatment with PCC gives 'A'. 'A' on reaction with sodium hypoiodite gives 'B', which on further heating with soda lime gives 'C. The compound 'C' is ______.
