Advertisements
Advertisements
Question
Derive an expression for the amount of heat given out or taken up, when its temperature falls or rises by t°C.
Solution
Let the mass of the body be ‘m’ and its specific heat capacity be ‘s’. It t°C is the rise in its temperature, then heat taken up by the body is calculated as below:
Since ‘s’ is the specific heat capacity of the body which means that the unit mass of the body for a rise of 1°C takes up ‘s’ amount of heat.
∴ Mass ‘m’ of the body for a rise of t°C will need heat
= m × s × t
= Mass of the body × Its specific heat × Rise in the temperature
= mst joule.
APPEARS IN
RELATED QUESTIONS
State two factors upon which the rate of emission of thermions depends.
State the effect of an increase of impurities on the melting point of ice.
What is the energy absorbed during the phase change called?
1 g ice of 0℃ melts to form 1 g water at 0℃. State whether the latent heat is absorbed or given out by ice.
Calculate the total amount of heat energy required to convert 100 g of ice at −10℃ completely into water at 100℃. Specific heat capacity of ice = 2.1 J g-1 K-1, specific heat capacity of water = 4.2 J g-1K-1, specific latent heat of ice = 336 J g-1.
During transformation of liquid phase to solid phase, the latent heat is ______.
Explain the following temperature Vs. time graph:
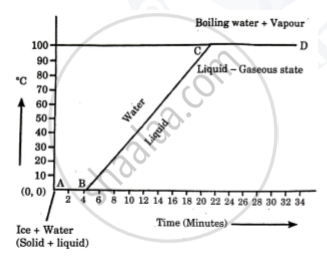
Explain the meaning of the term latent heat. State its S. I. unit.
Why do we feel much comfortable when we sit under a moving fan especially when our body is sweating?
What observation you will record and how will you determine the specific latent heat of fusion of ice?
Write the name.
Products obtained when sugar is heated.
1 kg of dry air at a temperature of 40 °C can hold a maximum of 49 g of water vapour.
Write scientific reason.
Even if boiling water is constantly heated, its temperature does not rise.
For the same mass of ice and ice-cold water, why does ice produce more cooling than ice-cold water?
Give some practical applications of specific latent heat of ice.
Calculate the amount of heat required to convert 200g of ice at 0°C into the water at 0°C Specific latent heat of fusion of ice = 336 Jg-1
2875 J of heat is required to melt 115 g of lead at its melting point. Calculate the specific latent heat capacity of fusion of lead.
