Advertisements
Advertisements
Questions
Describe the following:
Cannizzaro reaction
Write a note on Cannizzaro reaction.
Solution
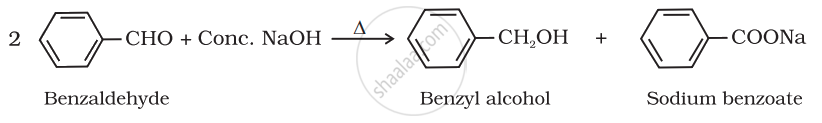
Aldehydes which do not have an α-hydrogen atom, undergo self-oxidation and reduction (disproportionation) reactions on heating with concentrated alkali. In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
\[\begin{array}{cc}
\phantom{.}\ce{H}\phantom{.......}\ce{H}\phantom{...............................}\ce{H}\phantom{................}\ce{O}\phantom{..}\\
\phantom{.......}\backslash\phantom{........}\backslash\phantom{..............................}|\phantom{...............}//\phantom{.......}\\
\ce{C = O + C = O + Conc.KOH ->[\Delta]H - C - OH + H - C}\\
\phantom{}/\phantom{........}/\phantom{..............................}|\phantom{................}\backslash\\
\phantom{.........}\ce{\underset{Formaldehyde}{H\phantom{........}H}\phantom{............................}\ce{\underset{Methanol}{H}}\phantom{.........}\ce{\underset{Potassium formate}{OK}}}\phantom{...}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Complete the following reactions:
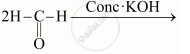
Write a note on the self oxidation-reduction reaction of an aldehyde with a suitable example.
Write the chemical equation for the reaction involved in Cannizzaro reaction.
Write the chemical equations to illustrate the following name reaction:
Cannizzaro’s reaction
Write the reactions involved in the following reactions: Clemmensen reduction
Write the product(s) in the following reactions

Write the equations involved in the following reactions:
Etard reaction
Complete the following reactions:
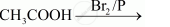
Complete the following reactions:
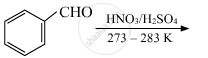
Write the chemical equations to illustrate the following name reactions:
Aldol condensation
Write the product formed when p-nitro chlorobenzene is heated with aqueous NaOH at 443K followed by acidification?
complete the following reaction:
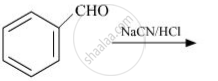
complete the following reaction:
\[\begin{array}{cc}
\phantom{...}\ce{CH3} \\
| \\
\phantom{.................}\ce{CH3-CH-COOH ->[(i) Br2/Red P4][(ii)H2O]}
\end{array}\]
Complete the following reaction:

The products obtained in the Cannizzaro reaction are
The key step in cannizzaro reaction in the inter molecular shift qf
Explain the following reaction:
Cannizzaro reaction
What happens when methanal undergoes cannizzaro reaction?
\[\begin{array}{cc}
\ce{D}\phantom{........................}\\
|\phantom{.........................}\\
\ce{2D - C = O + OH^- ->[Cannizzaro] X and Y}
\end{array}\]
(Y is alcohol, D is deuterium)
X and Y will have the structure:
In the Cannizzaro reaction given below:
\[\ce{2Ph-CHO ->[OH^-] Ph-CH2OH + PhC\overset{-}{O}_2}\]
the slowest step is:
Which of the following does not give Cannizzaro reaction?
Convert the following:
Benzene to m-nitrobenzaldehyde
Write the chemical reaction involved in Cannizzaro reaction of methanal.
