Advertisements
Advertisements
Question
Dilute NaOH can be tested with
(a) red litmus paper
(b) blue litmus paper
(c) lime water
(d) Na2CO3
Solution
(a) red litmus paper
Sodium hydroxide when dissolved in water undergoes the following
reaction:
NaOH(s) + aqs → Na+(aq) + OH−(aq)
The dissociated OH− ions turn moist red litmus paper blue.
APPEARS IN
RELATED QUESTIONS
| Column A | Column B | ||
| i | eosin | 1 | losing hydrogen |
| ii | oxidation | 2 | synthetic indicator |
| 3 | losing oxygen | ||
| 4 | natural indicator |
Write a word equation and then a balanced equation for the reaction taking place when:
Dilute hydrochloric acid reacts with magnesium ribbon.
Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. In which test tube will the fizzing occur more vigorously, and why?
Complete and balance the following chemical equations:
Zn (s) + HCI (aq) →
Complete and balance the following chemical equations:
CuO (s) + HCI (aq) →
What ions are present in the solutions of following substances? (write the symbols only)
Sodium hydroxide
What ions are present in the solutions of following substances? (write the symbols only)
Magnesium hydroxide
Hydrochloric acid reacts with a metal X to form a gas Y which burns with a 'pop' sound. Sodium hydroxide solution also reacts with the same metal X (on heating) to form the same gas Y.
Write the chemical equation of the reaction of metal X with (i) hydrochloric acid, and (ii) sodium hydroxide solution.
Name the product formed when Cl2 and H2 produced during the electrolysis of brine are made to combine.
Read the following statements:
I. When a red litmus paper is dipped into reaction mixture of a saponification reaction, it turns blue and the reaction is exothermic.
II. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is exothermic.
III. When a red litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
IV. When a blue litmus paper is dipped into reaction mixture of a saponification reaction, its colour does not change and the reaction is endothermic.
Which of the above statements are correct:
(A) I, and II
(B) II and III
(C) III and IV
(D) I and IV
When _______________ is passed through fresh lime water, it turns milky.
To protect tooth decay, we are advised to brush our teeth regularly. The nature of the tooth paste commonly used is
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
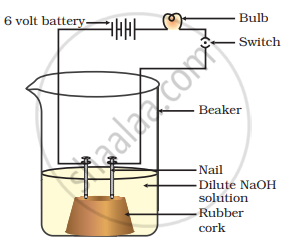
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
Which of the following is used for dissolution of gold?
Vinegar is ______ in taste.
Can we taste acids and bases to identify them?
______ & ______ metals do not react with HCl or HNO3.
What property do acids and bases have in common? Explain it with an example.
For each of the salt: A, B, C and D, suggest a suitable method of its preparation.
C is a soluble salt of copper.
