Advertisements
Advertisements
Question
Complete and balance the following chemical equations:
Zn (s) + HCI (aq) →
Solution
Zn(s) + 2HCI(aq) → ZnCI2(aq)+H2(g)
APPEARS IN
RELATED QUESTIONS
How is the concentration of hydroxide ions (OH−) affected when excess base is dissolved in a solution of sodium hydroxide?
Write a word equation and then a balanced equation for the reaction taking place when:
Dilute sulphuric acid reacts with aluminium powder.
What happens when an acid reacts with a metal carbonate? Explain with the help of an example. Write chemical equation of the reaction involved.
A substance X which is used as an antacid reacts with dilute hydrochloric acid to produce a gas Y which is used in one type of fire-extinguisher. Name the substance X and gas Y. Write a balanced equation for the chemical reaction which takes place.
Phenolphthalein is a synthetic type of indicator.
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
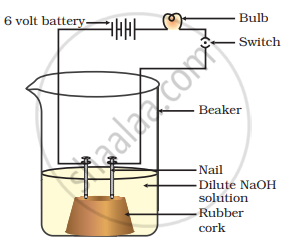
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
What happens when nitric acid is added to egg shell?
What are strong and weak acids? In the following list of acids, separate strong acids from weak acids.
Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
In the following schematic diagram for the preparation of hydrogen gas as shown in the figure, what would happen if following changes are made?

- In place of zinc granules, same amount of zinc dust is taken in the test tube
- Instead of dilute sulphuric acid, dilute hydrochloric acid is taken
- In place of zinc, copper turnings are taken
- Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
Which acid is present in milk?
