Advertisements
Advertisements
Question
Do all the electrons that absorb a photon come out as photoelectrons?
Solution
The photoelectric effect is the emission of electrons (called photo-electrons when light strikes a surface. To escape from the surface, the electron must absorb enough energy from the incident radiation to overcome the attraction of positive ions in the material of the surface.
The photoelectric effect is based on the principle of conservation of energy.
1. Two conducting electrodes, the anode (Q) and cathode (P) are enclosed in an evacuated glass tube as shown on next page.
2. The battery or other source of potential difference creates an electric field in the direction from anode to cathode.
3. Light of a certain wavelength or frequency falling on the surface of the cathode causes a current in the external circuit called photoelectric current.
4. As the potential difference increases, photoelectric current also increases till saturation is reached.
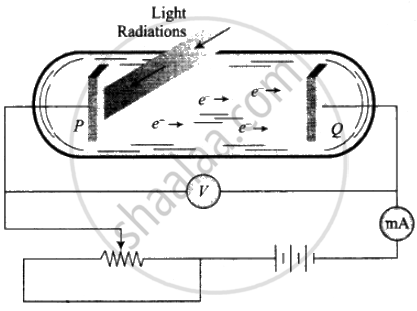
5. When polarity of the battery is reversed (i.e., plate Q is at negative potential w.r.t. plate P) electrons start moving back towards the cathode.
6. At a particular negative potential of plate Q, no electron will reach the plate Q and the current will become zero. This negative potential is called stopping potential denoted by V0. Maximum kinetic energy of photoelectrons in terms of stopping potential will therefore be Kmax = (|V0|) eV
So we conclude that in the photoelectric effect, we can observe that most electrons get scattered into the metal by absorbing a photon.
Therefore, all the electrons that absorb a photon don't come out as photoelectron. Only a few come out of metal whose energy becomes greater than the work function of the metal.
APPEARS IN
RELATED QUESTIONS
A hot body is placed in a closed room maintained at a lower temperature. Is the number of photons in the room increasing?
Should the energy of a photon be called its kinetic energy or its internal energy?
It is found that photosynthesis starts in certain plants when exposed to sunlight, but it does not start if the plants are exposed only to infrared light. Explain.
Calculate the momentum of a photon of light of wavelength 500 nm.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Calculate the number of photons emitted per second by a 10 W sodium vapour lamp. Assume that 60% of the consumed energy is converted into light. Wavelength of sodium light = 590 nm
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A sphere of radius 1.00 cm is placed in the path of a parallel beam of light of large aperture. The intensity of the light is 0.5 W cm−2. If the sphere completely absorbs the radiation falling on it, Show that the force on the sphere due to the light falling on it is the same even if the sphere is not perfectly absorbing.
Find the maximum magnitude of the linear momentum of a photoelectron emitted when a wavelength of 400 nm falls on a metal with work function 2.5 eV.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Answer the following question.
Plot a graph of photocurrent versus anode potential for radiation of frequency ν and intensities I1 and I2 (I1 < I2).
Consider a thin target (10–2 cm square, 10–3 m thickness) of sodium, which produces a photocurrent of 100 µA when a light of intensity 100W/m2 (λ = 660 nm) falls on it. Find the probability that a photoelectron is produced when a photons strikes a sodium atom. [Take density of Na = 0.97 kg/m3].
The graph shows the variation of photocurrent for a photosensitive metal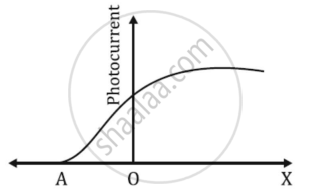
- What does X and A on the horizontal axis represent?
- Draw this graph for three different values of frequencies of incident radiation ʋ1, ʋ2 and ʋ3 (ʋ3 > ʋ2 > ʋ1) for the same intensity.
- Draw this graph for three different values of intensities of incident radiation I1, I2 and I3 (I3 > I2 > I1) having the same frequency.
