Advertisements
Advertisements
Question
Draw all the isomers (geometrical and optical) of [CoCl2(en)2]+.
Solution

trans [CoCl2(en)2]+
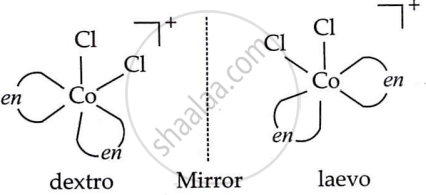
cis [CoCl2(en)2]+ optical isomers (d and l)
APPEARS IN
RELATED QUESTIONS
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex
Draw the geometrical isomers of complex [Pt(NH3)2Cl2].
Define the following term:
Anomers
Indicate the types of isomerism exhibited by the following complex and draw the structures for these isomers:
K[Cr(H2O)2(C2O4)2]
Indicate the types of isomerism exhibited by the following complex and draw the structures for these isomers:
[Co(en)3]Cl3
Indicate the types of isomerism exhibited by the following complex and draw the structures for these isomers:
[Co(NH3)5(NO2)](NO3)2
How many geometrical isomers are possible in the following coordination entity?
[Co(NH3)3Cl3]
Draw the structure of optical isomers of [PtCl2(en)2]2+.
What type of structural isomers are [Co(NH3)5 Br] SO4 and [Co(NH3)5 SO4]Br? Give a chemical test to distinguish the isomers.
Name the type of isomerism that the compound with molecular formula C3H6O2 exhibits. Represent the isomers.
Write the IUPAC name of [Co(en)2Cl2]+ ion and draw the structures of its geometrical isomers.
Answer the following question.
Write IUPAC name of the complex [Pt(en)2CI2]. Draw structures of geometrical isomers for this complex.
Draw the geometrical isomers of [Co(en)2Cl2]2+. Which geometrical isomer of [Co(en)2Cl2]2+ is not optically active and why?
Assertion: Addition of bromine water to 1-butene gives two optical isomers.
Reason: The product formed contains two asymmetric carbon atoms.
Indicate the type of isomerism exhibited by the following complex and draw the structure for this isomer:
[Pt(NH3)(H2O)Cl2]
Indicate the types of isomerism exhibited by the following complexes and draw the structure for these isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
Indicate the types of isomerism exhibited by the following complex and draw the structure for this isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
