Advertisements
Advertisements
Question
Indicate the types of isomerism exhibited by the following complex and draw the structures for these isomers:
[Co(NH3)5(NO2)](NO3)2
Solution
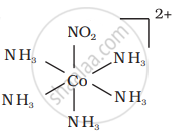
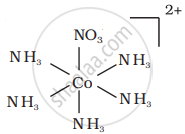
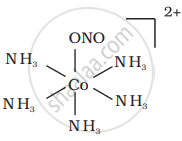
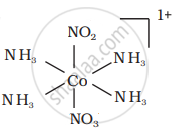
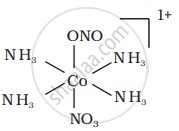
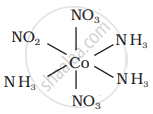
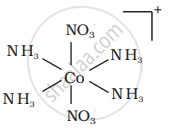
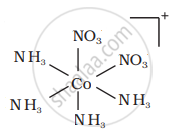
There are 10 possible isomers. (Geometrical, ionisation and linkage isomers)
 |
 |
 |
 |
 |
 |
 |
 |
 |
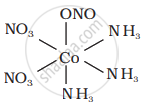 |
It can also show linkage isomerism.
[Co(NH3)5(NO2)](NO3)2 and [Co(NH3)5(ONO)](NO3)2
It can also show ionization isomerism.
[Co(NH3)5(NO2)](NO3)2 and [CO(NH3)5(NO3)](NO3)(NO2)
APPEARS IN
RELATED QUESTIONS
Explain cationic complexes and anionic complexes of co-ordination compounds.
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex
What type of isomerism is exhibited by the complex [Co(en)3]3+?
(en = ethane-1,2-diamine)
How many geometrical isomers are possible in the following coordination entity?
[Co(NH3)3Cl3]
Draw the structure of optical isomers of [PtCl2(en)2]2+.
Draw all the isomers (geometrical and optical) of [CoCl2(en)2]+.
Draw all the isomers (geometrical and optical) of [Co(NH3)2Cl2(en)]+.
What type of structural isomers are [Co(NH3)5 Br] SO4 and [Co(NH3)5 SO4]Br? Give a chemical test to distinguish the isomers.
Name the type of isomerism exhibited by the following pairs of compound:
(1) (C2H5)2NH and CH3-NH-C3H7
(2) 1 – butanol and 2 methyl-1 -propanol.
Name the type of isomerism shown by the following pair of compounds:
[CoCl(H2O)(NH3)4]Cl2 and [CoCl2(NH3)4]Cl.H2O
Write the IUPAC name of [Co(en)2Cl2]+ ion and draw the structures of its geometrical isomers.
Name the type of isomerism shown by the following pair of compounds:
[Cr(NH3)5Br]SO4 and [Cr(NH3)5SO4]Br
Which one of the following complexes shows optical isomerism? (en = ethylenediamine)
Indicate the types of isomerism exhibited by the following complexes and draw the structure for isomers:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers: [Pt(NH3)(H2O)Cl2]
Indicate the type of isomerism exhibited by the following complex and draw the structure for this isomer: \[\ce{[Pt(NH3)(H2O)Cl2]}\]
Indicate the types of isomerism exhibited by the following complex and draw the structure for this isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
Indicate the types of isomerism exhibited by the following complex and draw the structure for this isomer:
\[\ce{[Pt(NH3)(H2O)Cl2]}\]
