Advertisements
Advertisements
Question
For the pair phenol and cyclohexanol, answer the following:
Why is phenol more acidic than cyclohexanol?
Solution
Because the conjugate base of phenol, the phenoxide ion, is more stable due to resonance, phenol is more acidic than cyclohexanol.

APPEARS IN
RELATED QUESTIONS
Write the equation involved in the acetylation of Salicylic acid.
Give two reactions that show the acidic nature of phenol.
Intermolecular hydrogen bonding is strongest in ______.
Phenol is more acidic than alcohol because ____________.
Mark the correct order of decreasing acid strength of the following compounds.
| (a) | 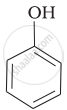 |
| (b) | 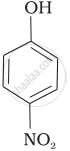 |
| (c) | 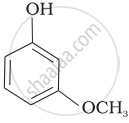 |
| (d) | 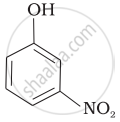 |
| (e) | 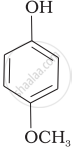 |
Phenol is used in the manufacture of
Arrange the following in the increasing order of their property indicated:
4-Nitrobenzoic acid, benzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxy benzoic acid (Acid strength)
In the following compounds:
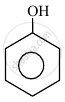 |
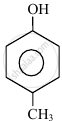 |
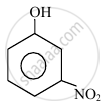 |
 |
| (I) | (II) | (III) | (IV) |
The order to acidity is ______.
Give the structure of the product you would expect when the following alcohol reacts with SOCl2.
Butan-1-ol
Compare acidity of phenol with that of ethanol.
