Advertisements
Advertisements
Question
Give an equation state for an isochoric process.
Solution
The equation of state for an isochoric process is given by
P = `((μ"R")/"V") "T"`
where `((μ"R")/"V")` = Constant
We can infer that the pressure is directly proportional to temperature. This implies that the P-T graph for an isochoric process is a straight line passing through the origin.
APPEARS IN
RELATED QUESTIONS
An ideal gas is taken through an isothermal process. If it does 2000 J of work on its environment, how much heat is added to it?
Explain the thermodynamics of the isobaric process.
When a cycle tyre suddenly bursts, the air inside the tyre expands. This process is ____________.
Apply first law for an isothermal process.
Draw the PV diagram for the isobaric process.
Can the given heat energy be completely converted to work in a cyclic process? If not, when can the heat can completely converted to work?
Consider the following cyclic process consist of isotherm, isochoric and isobar which is given in the figure.
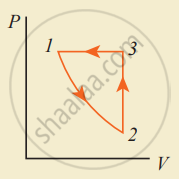
Draw the same cyclic process qualitatively in the V-T diagram where T is taken along the x-direction and V is taken along the y-direction. Analyze the nature of heat exchange in each process.
A monoatomic gas of pressure p having volume V expands isothermally to a volume 2V and then adiabatically to a volume 16V. The final pressure of the gas is ____________.
`("ratio of specific heats" = 5/3)`
In which of the following processes, beat is neither absorbed nor released by a system?
An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas?
