Advertisements
Advertisements
Question
Explain the thermodynamics of the isobaric process.
Solution
- A thermodynamic process that is carried out at constant pressure i.e., Δp = 0 is called the isobaric process.
- For an isobaric process, none of the quantities ΔU, Q, and W is zero.
- The temperature of the system changes, i.e., ΔT ≠ 0.
- The energy exchanged is used to do work as well as to change internal energy causing an increase in temperature. Thus, Q = ΔU + W.
- As work is done volume changes during the process.
- Heat exchanged in case of an isobaric process:
a. Consider an ideal gas undergoing volume expansion at constant pressure.
b. If Vi and Ti are its volume and temperature in the initial state of a system and Vf and Tf are its final volume and temperature respectively, the work done in the expansion is given by,
W = pdV = p(Vf – Vi) = nR(Tf – Ti) ….(1)
c. Also, the change in the internal energy of a system is given by, ΔU = nCVΔT = nCV(Tf – Ti) ….(2)
Where, CV is the specific heat at constant volume, and ΔT = (Tf – Ti) is the change in its temperature during the isobaric process.
d. According to the first law of thermodynamics, the heat exchanged is given by, Q = ΔU + W
Using equations (1) and (2) we get,
Q = nCV(Tf – Ti) + nR(Tf – Ti)
∴ Q = (nCV + nR) (Tf – Ti)
∴ Q = nCp(Tf – Ti) .............(∵ Cp = CV + R)
Where, Cp is the specific heat at constant pressure. - The p-V diagram for an isobaric process is called isobar. It is shown in the figure below.

APPEARS IN
RELATED QUESTIONS
What is a thermodynamic process?
Draw a p-V diagram showing positive work with varying pressure.
Explain the cyclic process.
Explain graphically (i) positive work with varying pressure, (ii) negative work with varying pressure, and (iii) positive work at constant pressure.
Write a note on free expansion.
Explain work done during a thermodynamic process.
Explain the thermodynamics of the isochoric process.
When a cycle tyre suddenly bursts, the air inside the tyre expands. This process is ____________.
When you exercise in the morning, by considering your body as a thermodynamic system, which of the following is true?
The V-T diagram of an ideal gas which goes through a reversible cycle A→B→C→D is shown below. (Processes D→A and B→C are adiabatic)
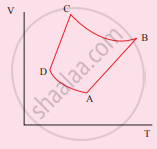
The corresponding PV diagram for the process is (all figures are schematic)
In an isochoric process, we have ____________.
Apply first law for an isobaric process.
Give the equation of state for an adiabatic process.
Draw the PV diagram for the isothermal process.
Draw the PV diagram for the adiabatic process.
Can the given heat energy be completely converted to work in a cyclic process? If not, when can the heat can completely converted to work?
Explain in detail an adiabatic process.
Explain in detail the isochoric process.
Consider the following cyclic process consist of isotherm, isochoric and isobar which is given in the figure.
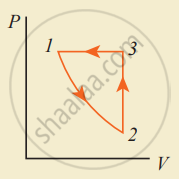
Draw the same cyclic process qualitatively in the V-T diagram where T is taken along the x-direction and V is taken along the y-direction. Analyze the nature of heat exchange in each process.
An ideal gas is taken in a cyclic process as shown in the figure. Calculate
- work done by the gas
- work done on the gas
- Net work done in the process

For a given ideal gas 6 × 105 J heat energy is supplied and the volume of gas is increased from 4 m3 to 6 m3 at atmospheric pressure. Calculate
- the work done by the gas
- change in internal energy of the gas
- graph this process in PV and TV diagram
A thermodynamic system undergoes cyclic process ABCDA as shown in the figure. The work done by the system is ______
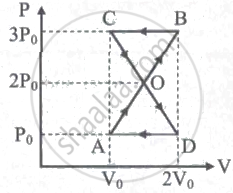
An ideal gas is made to go from a state A to stale B in the given two different ways (see figure) (i) an isobaric and then an isochoric process and (ii) an isochoric and then an isobaric process. The work done by gas in the two processes are W1 and W2 respectively. Then,

Assertion: Equal volumes of monatomic and polyatomic gases are adiabatically compressed separately to equal compression ratio `("P"_2/"P"_1)`. Then monatomic gas will have greater final volume.
Reason: Among ideal gases, molecules of a monatomic gas have the smallest number of degrees of freedom.
Which of the following processes is reversible?
Consider P-V diagram for an ideal gas shown in figure.
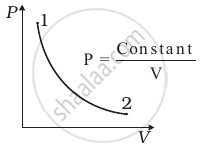
Out of the following diagrams (figure), which represents the T-P diagram?
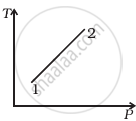 (i) |
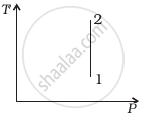 (ii) |
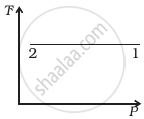 (iii) |
 (iv) |
Explain how can a gas be expanded at constant temperature.
