Advertisements
Advertisements
Question
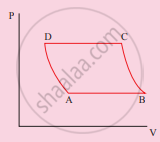
The V-T diagram of an ideal gas which goes through a reversible cycle A→B→C→D is shown below. (Processes D→A and B→C are adiabatic)
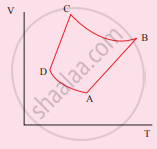
The corresponding PV diagram for the process is (all figures are schematic)
Options
Solution
APPEARS IN
RELATED QUESTIONS
Explain why The climate of a harbour town is more temperate than that of a town in a desert at the same latitude.
Heating a gas in a constant volume container is an example of which process?
Draw a p-V diagram of the reversible process.
Derive the work done in an adiabatic process.
Explain the isobaric process and derive the work done in this process.
An ideal gas is made to go from a state A to stale B in the given two different ways (see figure) (i) an isobaric and then an isochoric process and (ii) an isochoric and then an isobaric process. The work done by gas in the two processes are W1 and W2 respectively. Then,

In which of the following processes, beat is neither absorbed nor released by a system?
Assertion: Equal volumes of monatomic and polyatomic gases are adiabatically compressed separately to equal compression ratio `("P"_2/"P"_1)`. Then monatomic gas will have greater final volume.
Reason: Among ideal gases, molecules of a monatomic gas have the smallest number of degrees of freedom.
An ideal gas is taken through a cyclic process ABCDA as shown in figure. The net work done by the gas during the cycle is ______.
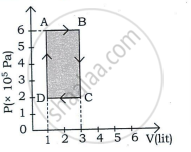
In a cyclic process, if ΔU = internal energy, W = work done, Q = Heat supplied then ______.