Advertisements
Advertisements
Question
Draw the PV diagram for the adiabatic process.
Solution

APPEARS IN
RELATED QUESTIONS
Differentiate between the reversible and irreversible processes.
Explain work done during a thermodynamic process.
Explain the thermodynamics of the isochoric process.
When a cycle tyre suddenly bursts, the air inside the tyre expands. This process is ____________.
Draw the PV diagram for the isothermal process.
What is meant by a reversible and irreversible processes?
Can the given heat energy be completely converted to work in a cyclic process? If not, when can the heat can completely converted to work?
Consider the following cyclic process consist of isotherm, isochoric and isobar which is given in the figure.
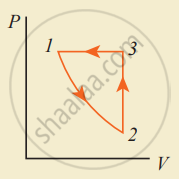
Draw the same cyclic process qualitatively in the V-T diagram where T is taken along the x-direction and V is taken along the y-direction. Analyze the nature of heat exchange in each process.
An ideal gas is expanded isothermally from volume V1 to volume V2 and then compressed adiabatically to original volume V1. If the initial pressure is P1, the final pressure is P3 and net work done is W, then ____________.
An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas?
