Advertisements
Advertisements
Question
What is meant by a reversible and irreversible processes?
Solution
Reversible processes: A thermodynamic process can be considered reversible only if it possible to retrace the path in the opposite direction in such a way that the system and surroundings pass through the same states as in the initial, direct process.
Irreversible processes: All natural processes are irreversible. Irreversible processes cannot be plotted in a PV diagram, because these processes cannot have unique values of pressure, the temperature at every stage of the process.
APPEARS IN
RELATED QUESTIONS
Answer in brief.
Why should a Carnot cycle have two isothermal two adiabatic processes?
What is a thermodynamic process?
Explain work done during a thermodynamic process.
Apply first law for an isothermal process.
Draw the PV diagram for the isothermal process.
Draw the PV diagram for the isochoric process.
Consider the following cyclic process consist of isotherm, isochoric and isobar which is given in the figure.
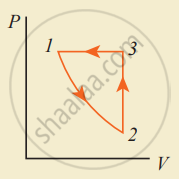
Draw the same cyclic process qualitatively in the V-T diagram where T is taken along the x-direction and V is taken along the y-direction. Analyze the nature of heat exchange in each process.
The work done on the system in changing the state of a gas adiabatically from equilibrium state A to equilibrium state B is 22.4 J. If the gas is taken from state A to B through another process in which the net heat absorbed by the system is 15.5 cal, then the net work done by the system in the latter case is ______.
( l cal = 4.2 J)
Consider P-V diagram for an ideal gas shown in figure.
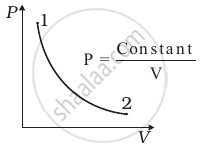
Out of the following diagrams (figure), which represents the T-P diagram?
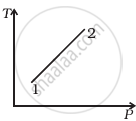 (i) |
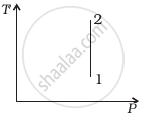 (ii) |
 (iii) |
 (iv) |
In a certain thermodynamical process, the pressure of a gas depends on its volume as kV3. The work done when the temperature changes from 100°C to 300°C will be ______ nR, where n denotes number of moles of a gas.
