Advertisements
Advertisements
Question
Explain in detail the isochoric process.
Solution
Isochoric process: This is a thermodynamic process in which the volume of the system is kept constant. But pressure, temperature and internal energy continue to be variables.
The pressure-volume graph for an isochoric process is a vertical line parallel to the pressure axis as shown in Figure.

Isochoric process with increased pressure
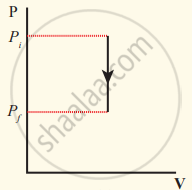
Isochoric process with decreased pressure

Isochoric process
P = `((μ"R")/"V")"T"` ...............(1)
where `((μ"R")/"V")` = constant
We can infer that the pressure is directly proportional to temperature. This implies that the P-T graph for an isochoric process is a straight line passing through the origin. If gas goes from the state (Pi, Ti) to (Pf, Tf) at constant volume, then the system satisfies the following equation
`"P"_"i"/"T"_"i" = "P"_"f"/"T"_"f"` .....,,,(2)
For an isochoric processes, ∆V = 0 and W = 0. Then the first law becomes
∆U = 0 .................(3)
Implying that the heat supplied is used to increase only the internal energy. As a result the temperature increases and pressure also increases.
Suppose a system loses heat to the surroundings through conducting walls by keeping the volume constant, then its internal energy decreases. As a result the temperature decreases; the pressure also decreases.
APPEARS IN
RELATED QUESTIONS
Draw a p-V diagram showing negative work with varying pressure.
Explain work done during a thermodynamic process.
When a cycle tyre suddenly bursts, the air inside the tyre expands. This process is ____________.
Give an expression for work done in an isothermal process.
Give an equation state for an isochoric process.
Explain in detail the isothermal process.
Explain the isobaric process and derive the work done in this process.
A monoatomic gas of pressure p having volume V expands isothermally to a volume 2V and then adiabatically to a volume 16V. The final pressure of the gas is ____________.
`("ratio of specific heats" = 5/3)`
An ideal gas A and a real gas B have their volumes increased from V to 2V under isothermal conditions. The increase in internal energy ____________.
We consider a thermodynamic system. If `Delta"U"` represents the increase in its internal energy and W the work done by the system, which of the following statements is true?
