Advertisements
Advertisements
Question
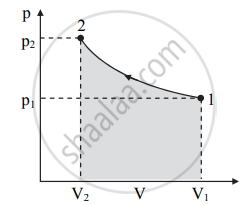
Draw a p-V diagram showing negative work with varying pressure.
Solution
p-V diagram showing negative work with varying pressure:
RELATED QUESTIONS
A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in Figure

Its volume is then reduced to the original value from E to F by an isobaric process. Calculate the total work done by the gas from D to E to F
What is a thermodynamic process?
Draw a p-V diagram of the reversible process.
Draw a p-V diagram of the irreversible process.
Draw a p-V diagram showing positive work with varying pressure.
Differentiate between the reversible and irreversible processes.
Explain the cyclic process.
3 mole of a gas at temperature 400 K expands isothermally from an initial volume of 4 litres to a final volume of 8 litres. Find the work done by the gas. (R = 8.31 J mol-1 K-1)
Explain graphically (i) positive work with varying pressure, (ii) negative work with varying pressure, and (iii) positive work at constant pressure.
Write a note on free expansion.
Explain the thermodynamics of the isobaric process.
In an isochoric process, we have ____________.
Give an expression for work done in an isothermal process.
Apply first law for an isobaric process.
Give the equation of state for an adiabatic process.
Derive the work done in an isothermal process.
Explain in detail the isochoric process.
In an adiabatic expansion of the air, the volume is increased by 4%, what is the percentage change in pressure? (For air γ = 1.4)
An ideal gas is taken in a cyclic process as shown in the figure. Calculate
- work done by the gas
- work done on the gas
- Net work done in the process

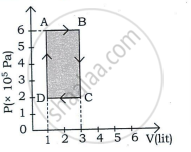
A thermodynamic system undergoes cyclic process ABCDA as shown in the figure. The work done by the system is ______
An ideal gas is made to go from a state A to stale B in the given two different ways (see figure) (i) an isobaric and then an isochoric process and (ii) an isochoric and then an isobaric process. The work done by gas in the two processes are W1 and W2 respectively. Then,

An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas?
We consider a thermodynamic system. If `Delta"U"` represents the increase in its internal energy and W the work done by the system, which of the following statements is true?
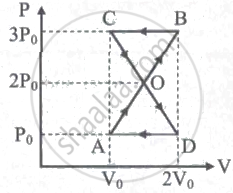
An ideal gas is taken through a cyclic process ABCDA as shown in figure. The net work done by the gas during the cycle is ______.
Explain how can a gas be expanded at constant temperature.
In a cyclic process, if ΔU = internal energy, W = work done, Q = Heat supplied then ______.
