Advertisements
Advertisements
Question
Give reasons Although –NH2 is o/p directing group, yet aniline on nitration gives a significant amount of m-nitroaniline
Solution
Nitration is usually carried out with a mixture of concentrated HNO3 and concentrated H2SO4. In the presence of these acids, most of aniline gets protonated to form anilinium ion. Therefore, in presence of acids, the reaction mixture consists of aniline and anilinium ion. Nitration of aniline due to steric hindrance at ortho position, mainly gives para nitroaniline and the nitration of anilinium ion gives m-nitroaniline. In actual practice, approximately 1:1 mixture of p-nitroaniline: m-nitroaniline is obtained.
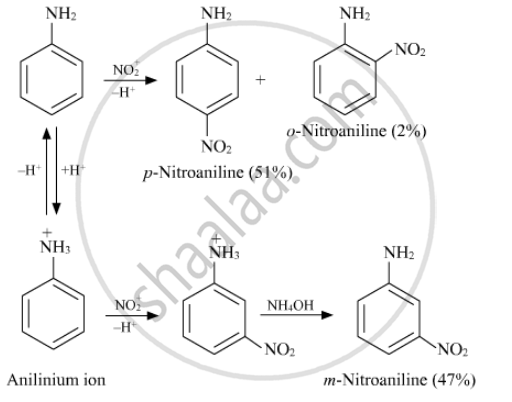
Thus, nitration of aniline gives a substantial amount of m-nitroaniline due to protonation of the amino group.
APPEARS IN
RELATED QUESTIONS
Write the IUPAC name of the following compound and classify it as primary, secondary and tertiary amine.
m−BrC6H4NH2
Give one chemical test to distinguish between the following pair of compounds.
Secondary and tertiary amines
Account for the following:
Ethylamine is soluble in water whereas aniline is not.
How will you convert Hexanenitrile into 1-aminopentane
Accomplish the following conversions - Aniline to benzyl alcohol.
Complete the following reactions:
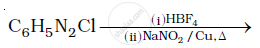
Do the following conversions in not more than two steps :
Ethyl benzene to Benzoic acid
Do the following conversions in not more than two steps :
Propanone to Propene
Using IUPAC norms write the formula of Hexaamminecobalt (III) sulphate.
Do the following conversions in not more than two steps:

