Advertisements
Advertisements
Question
Is it easier to take out a nucleon (a) from carbon or from iron (b) from iron or from lead?
Solution
Binding energy per nucleon of a nucleus is defined as the energy required to break-off a nucleon from it.
(a) As the binding energy per nucleon of iron is more than that of carbon, it is easier to take out a nucleon from carbon than iron.
(b) As the binding energy per nucleon of iron is more than that of lead. Therefore, it is easier to take out a nucleon from lead as compared to iron.
APPEARS IN
RELATED QUESTIONS
Obtain the binding energy of the nuclei `""_26^56"Fe"` and `""_83^209"Bi"` in units of MeV from the following data:
`"m"(""_26^56"Fe")` = 55.934939 u
`"m"(""_83^209"Bi")`= 208.980388 u
The neutron separation energy is defined as the energy required to remove a neutron from the nucleus. Obtain the neutron separation energies of the nuclei `""_20^41"Ca"` and `""_13^27 "Al"` from the following data:
`"m"(""_20^40"Ca")` = 39.962591 u
`"m"(""_20^41"Ca")` = 40.962278 u
`"m"(""_13^26"Al")` = 25.986895 u
`"m"(""_13^27"Al")` = 26.981541 u
Define half-life of a radioactive substance
Define the terms (i) half-life (T1/2) and (ii) average life (τ). Find out their relationships with the decay constant (λ).
What characteristic property of nuclear force explains the constancy of binding energy per nucleon (BE/A) in the range of mass number ‘A’ lying 30 < A < 170?
In which of the following decays the atomic number decreases?
(a) α-decay
(b) β+-decay
(c) β−-decay
(d) γ-decay
Binding energy per nucleon for helium nucleus (2 He) is 7.0 MeV Find value of mass defect for helium nucleus
In a nuclear reactor, what is the function of:
(i) The moderator
(ii) The control rods
(iii) The coolant
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
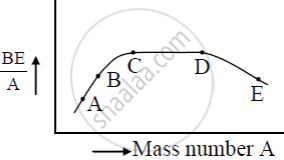
Answer the following question.
Draw the curve showing the variation of binding energy per nucleon with the mass number of nuclei. Using it explains the fusion of nuclei lying on the ascending part and fission of nuclei lying on the descending part of this curve.
Calculate the binding energy of an alpha particle given its mass to be 4.00151 u.
An electron in hydrogen atom stays in its second orbit for 10−8 s. How many revolutions will it make around the nucleus at that time?
Determine the binding energy per nucleon of the americium isotope \[\ce{_95^244Am}\], given the mass of \[\ce{_95^244Am}\] to be 244.06428 u.
A body's centre of mass
Mx and My denote the atomic masses of the parent and the daughter nuclei respectively in a radioactive decay. The Q-value for a β– decay is Q1 and that for a β+ decay is Q2. If m e denotes the mass of an electron, then which of the following statements is correct?
Heavy stable nucle have more neutrons than protons. This is because of the fact that ______.
Define binding energy per nucleon.
What is meant by “binding energy per nucleon” of a nucleus?
