Advertisements
Advertisements
Question
Justify that the following reaction is redox reaction; identify the species oxidized/reduced, which acts as an oxidant and which acts as a reductant.
\[\ce{I2_{(aq)} + 2S2O^{2-}_{3(aq)}->S4O^{2-}_{6(aq)} + 2I^-_{ (aq)}}\]
Solution
\[\ce{I2_{(aq)} + 2S2O^{2-}_{3(aq)}->S4O^{2-}_{6(aq)} + 2I^-_{ (aq)}}\]
- Write oxidation number of all the atoms of reactants and products.
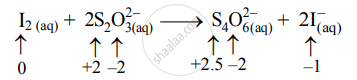
- Identify the species that undergoes a change in oxidation number.
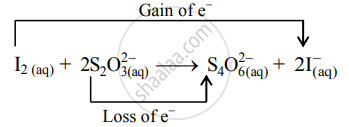
- The oxidation number of S increases from +2 to +2.5 and that of I decreases from 0 to –1. Because oxidation number of one species increases and that of the other decreases, the reaction is a redox reaction.
- The oxidation number of S increases by loss of electrons and therefore, S is a reducing agent and itself is oxidized. On the other hand, the oxidation number of I decreases by a gain of electrons, and therefore, I is an oxidizing agent and itself is reduced.
Result:
- The given reaction is a redox reaction.
- Oxidant/oxidizing agent (Reduced species): I2
- Reductant/reducing agent (Oxidized species): \[\ce{S2O^2-_3}\]
APPEARS IN
RELATED QUESTIONS
Choose the correct option.
The coefficients p, q, r, s in the reaction \[\ce{{p}Cr2O7^{2Θ} + {q}Fe^{2⊕}->{r}Cr^{3⊕} + {s}Fe^{3⊕} + H2O}\] respectively are:
Choose the correct option.
Which of the following halogens does always show oxidation state -1?
In which reaction does nitrogen exhibit variation of oxidation state from –3 to +5?
Calculate the oxidation number of the underlined atom.
H2SO4
Calculate the oxidation number of the underlined atom.
H3PO3
Calculate the oxidation number of the underlined atom.
NaH2PO4
What is oxidation?
Identify the following pair of species is in its oxidized state.
Mg/Mg2+
Justify the following reaction as a redox reaction.
\[\ce{2Na_{(s)} + S_{(s)}->Na2S_{(s)}}\]
Find out the oxidizing and reducing agents.
Provide the stock notation for the following compound:
HAuCl4
Provide the stock notation for the following compound:
Tl2O
Provide the stock notation for the following compound:
CuO
Assign oxidation number atom in the following species.
Na2S2O3
Which of the following redox couple is a stronger oxidizing agent?
Cl2 (E0 = 1.36 V) and Br2 (E0 = 1.09 V)
Which of the following redox couple is a stronger reducing agent?
Li (E0 = - 3.05 V) and Mg (E0 = - 2.36 V)
Which of the following redox couple is a stronger reducing agent?
Zn (E0 = - 0.76 V) and Fe (E0 = - 0.44 V)
Complete the following table:
Assign oxidation number to the underlined species and write Stock notation of compound
| Compound | Oxidation number | Stock notation |
| AuCl3 | ||
| SnCl2 | ||
| \[\ce{\underline{{V}}_2O^{4-}_{7}}\] | ||
| \[\ce{\underline{{Pt}}Cl^2-_6}\] | ||
| H3AsO3 |
Oxidation state of Xe in XeOF4 is ____________.
The oxidation number of oxygen in peroxides is ____________.
Which of the following is CORRECT?
\[\ce{H3PO4_{(aq)} + 3KOH_{(aq)} -> K3PO4_{(aq)} + 3H2O_{(l)}}\]
In the reaction,
\[\ce{MnO^{-1}_4 (aq) + Br^{-1}(aq) -> MnO2(s) + BrO^{-1}_3(aq)}\]
the correct change in oxidation number of the species involved is ______.
Which of the following is NOT an example of redox reaction?
In the complex [Co(en)3]Cl3, ____________.
In the following reaction, the oxidation number of Cr changes.
\[\ce{ClO^-_{( aq)} + Cr(OH)^-_{4(aq)} -> CrO^{2-}_{4(aq)} + Cl^-_{( aq)} (basic)}\]
Match the following.
| Compound | Oxidation no. of underlined element |
| i. \[\ce{\underline{C}_4H4O^{2-}_6}\] | a. +2.5 |
| ii. \[\ce{\underline{N}_3H}\] | b. +1.5 |
| iii. \[\ce{Mg2\underline{P}_2O7}\] | c. +5 |
| iv. \[\ce{Na2\underline{S}_4O6}\] | d. `-1//3` |
The sum of oxidation number of all atoms in \[\ce{S2O^{2-}_3}\] ion is ______.
Which among the following pair of elements show highest oxidation state +7 in their different compounds?
Which of the following explanation is correct about the given below reaction?
\[\ce{Cr2O^2-_7 + H2O -> 2CrO^2-_4 + 2H+}\]
