Advertisements
Advertisements
Question
Justify that the following reaction is redox reaction; identify the species oxidized/reduced, which acts as an oxidant and which acts as a reductant.
\[\ce{HF_{(aq)} + {OH}^-_{ (aq)}->H2O_{(l)} + {F}^ -_{ (aq)}}\]
Solution
\[\ce{HF_{(aq)} + {OH}^-_{ (aq)}->H2O_{(l)} + {F}^ -_{ (aq)}}\]
- Write oxidation number of all the atoms of reactants and products
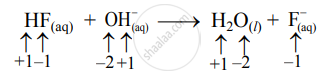
- Since the oxidation numbers of all the species remain the same, this is NOT a redox reaction.
Result:
The given reaction is NOT a redox reaction.
APPEARS IN
RELATED QUESTIONS
Choose the correct option.
A compound contains atoms of three elements A, B, and C. If the oxidation state of A is +2, B is +5 and that of C is -2, the compound is possibly represented by
Choose the correct option.
The coefficients p, q, r, s in the reaction \[\ce{{p}Cr2O7^{2Θ} + {q}Fe^{2⊕}->{r}Cr^{3⊕} + {s}Fe^{3⊕} + H2O}\] respectively are:
Choose the correct option.
Oxidation number of carbon in H2CO3 is
Choose the correct option.
Which is the correct stock notation for manganese dioxide?
Calculate the oxidation number of the underlined atom.
K2C2O4
Identify the following pair of species is in its oxidized state.
Mg/Mg2+
Justify the following reaction as a redox reaction.
\[\ce{2Na_{(s)} + S_{(s)}->Na2S_{(s)}}\]
Find out the oxidizing and reducing agents.
Provide the stock notation for the following compound:
FeO
Provide the stock notation for the following compound:
Fe2O3
Provide the stock notation for the following compound:
MnO
Provide the stock notation for the following compound:
CuO
Which of the following redox couple is a stronger oxidizing agent?
Cl2 (E0 = 1.36 V) and Br2 (E0 = 1.09 V)
Which of the following redox couple is a stronger oxidizing agent?
\[\ce{MnO^Θ_4}\](E0 = 1.51 V) and \[\ce{Cr2O^{2Θ}_7}\](E0 = 1.33 V)
The following statements are CORRECT, EXCEPT:
What is the oxidation number of As in H3AsO3?
Which of the following is NOT an example of redox reaction?
Oxidation state of nitrogen in nitric oxide is ______.
Stock notations are used to specify the oxidation numbers of ____________.
The sum of oxidation number of all atoms in \[\ce{S2O^{2-}_3}\] ion is ______.
Carbon is present in highest oxidation number in ______.
The oxidation number of Cr in \[\ce{Cr(OH)^-_4}\] ion is ______.
The oxidation number of phosphorous in Ba(H2PO2)2 is ______.
What is the oxidation number of Cr in K2Cr2O7?
The oxidation number of oxygen in oxygen difluoride (OF2) and dioxygen difluoride (O2F2) respectively is ______.
