Advertisements
Advertisements
Question
Monomer of natural rubber is __________
Solution
Monomer of natural rubber is isoprene
APPEARS IN
RELATED QUESTIONS
Write any ‘two' uses of terylene.
Explain the following term: Homopolymers
Based on molecular forces, what type of polymer is neoprene?
Write the structures of the monomers used for getting the following polymers
Melamine – formaldehyde polymer
Draw the structures of veronal and thymine.
Write the monomers of the following polymer :
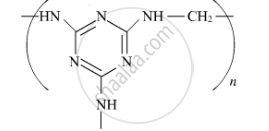
Choose the correct option from the given alternatives.
Which of the following is made up of polyamides?
Answer the following in one sentence.
What type of intermolecular force leads to high-density polymer?
Answer the following in one sentence.
Identify thermoplastic and thermosetting plastic from the following:
- PET
- Urea formaldehyde resin
- Polythene
- Phenol formaldehyde resin
Answer the following.
Write the reaction of the formation of Terylene.
Answer the following.
Name the polymer type in which following linkage is present.
\[\begin{array}{cc}\ce{- C - O -}\\||\phantom{.....}\\
\ce{O\phantom{.....}}\end{array}\]
Answer the following.
Match the following pairs:
| Name of polymer | Monomer |
| 1. Teflon | a. CH2 = CH2 |
| 2. PVC | b. CF2 = CF2 |
| 3. Polyester | c. CH2 = CHCl |
| 4. Polythene | d. C6H5OH and HCHO |
| 5. Bakelite | e. Dicarboxylic acid and polyhydoxyglycol |
Write the reaction involved in the formation of:
Bakelite
Attempt the following:
What is meant by LDP and HDP? Mention the basic difference between the same with suitable examples.
Attempt the following:
Write preparation, properties and uses of Teflon.
Answer the following.
Is synthetic rubber better than natural rubber? If so, in what respect?
Answer the following.
Write main specialities of Buna-S, Neoprene rubber?
Write the name of the catalyst used for preparation of high density polythene polymer.
Write preparation of low density polythene.
Write chemical reaction for preparation of the following.
Buna-S
Explain vulcanization of rubber.
Which among the following polymers is obtained from styrene and 1-3-butadiene?
Identify additional polymers from the following.
I. \[\begin{array}{cc}
\ce{-(CH2 - CH -)_{{n}}}\\
\phantom{....}|\\
\phantom{.......}\ce{C6H5}
\end{array}\]
II. \[\ce{-(CH2 - CH = CH - CH2 -)_{{n}}}\]
III. \[\ce{-(CO(CH2)4 - CONH(CH2)6NH -)_{{n}}}\]
IV.
![]()
How many isoprene units are present in abscisic acid?
The INCORRECT match for the polymer with its application is:
Select the CORRECT match for both the polymers.
Which of the following polymers is obtained from chloroprene?
Novolac is obtained from ____________.
Identify the polymer obtained by polymerization of n moles of acrylonitrile.
Which among the following polymers is obtained from CH2 = CH – CN by polymerisation?
Identify the monomers used in the preparation of Novolac.
Identify the catalyst used in the manufacture of high density polythene.
Which among the following monomers is used to prepare Teflon?
Which of the following pair of compounds is used as monomers for bakelite?
Which of the following polymer is used to make blankets?
Which among the following polymers is used to manufacture chemical containers?
Which of the following polymers is used as insulation for cables?
The monomer of Teflon is ______.
Which of the following polymer has ester linkage?
Trans - form of poly isoprene is:-
Which of the following polymers is synthesized using a free radical polymerisation technique?
Which of the following polymer is used for manufacturing of buckets, dustbins, pipes, etc?
Which of the following is a polymer of enzyme?
Write the structure of isoprene and the polymer obtained from it.
Write the structure and name of monomer of Natural rubber.
Name and draw structure of the repeating unit in natural rubber.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Write the structure of isoprene and the polymer obtained from it.
Write the structure of isoprene and the polymer obtained from it.
