Advertisements
Advertisements
Question
The figure shows two vessels with adiabatic walls, one containing 0.1 g of helium (γ = 1.67, M = 4 g mol−1) and the other containing some amount of hydrogen (γ = 1.4, M = 2 g mol−1). Initially, the temperatures of the two gases are equal. The gases are electrically heated for some time during which equal amounts of heat are given to the two gases. It is found that the temperatures rise through the same amount in the two vessels. Calculate the mass of hydrogen.
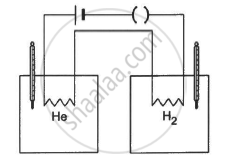
Solution
Given:
Mass of He, mHe = 0.1 g
γ1 = 1.67
Molecular weight of He, MHe = 4 g/mol
MH2 = ?
MH2 = 2 g/mol
γ2 = 1.4
Since it is an adiabatic environment and the system is not dong any external work, the amount of heat given will be used up entirely to raise its internal energy.
For He, dQ = dU = nCvdT ...(i)
`= "m"/4 xx "R"/(gamma-1) xx "d""T"`
`= 0.1 /4 xx "R"/((1.67 -1)) xx "d""T"`
For H2, dQ =dU = nCvdT ...(ii)
=`"m"/2 xx "R"/(gamma-1) xx "d""T"`
=`"m"/2 xx "R"/(1.4-1) xx "d""T"`
where m is the required mass of H2.
Since equal amount of heat is given to both gases; so dQ is same in both eq (i) and (ii), we get
`0.1/4 xx "R"/0.67""d""T"`
`= "m"/2 xx "R"/0.4 xx"d""T"`
`= m = 0.1/2 xx 0.4 /0.67`
`=> m =0.0298 ≈ 0.03 "g"`
APPEARS IN
RELATED QUESTIONS
Given below are densities of some solids and liquids. Give rough estimates of the size of their atoms:
| Substance | Atomic Mass (u) | Density (103 Kg m-3) |
| Carbon (diamond) | 12.01 | 2.22 |
| Gold | 197.00 | 19.32 |
| Nitrogen (liquid) | 14.01 | 1.00 |
| Lithium | 6.94 | 0.53 |
| Fluorine (liquid) | 19.00 | 1.14 |
[Hint: Assume the atoms to be ‘tightly packed’ in a solid or liquid phase, and use the known value of Avogadro’s number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few Å].
Does a gas have just two specific heat capacities or more than two? Is the number of specific heat capacities of a gas countable?
Can we define specific heat capacity at constant temperature?
Can we define specific heat capacity for an adiabatic process?
Does a solid also have two kinds of molar heat capacities Cp and Cv? If yes, is Cp > Cv? Or is Cp − Cv = R?
Show that the slope of the p−V diagram is greater for an adiabatic process compared to an isothermal process.
Two samples A and B are initially kept in the same state. Sample A is expanded through an adiabatic process and the sample B through an isothermal process. The final volumes of the samples are the same. The final pressures in A and B are pA and pBrespectively.
Let ∆Wa and ∆Wb be the work done by the systems A and B, respectively, in the previous question.
Consider the processes A and B shown in the figure. It is possible that
An ideal gas expands from 100 cm3 to 200 cm3 at a constant pressure of 2.0 × 105 Pa when 50 J of heat is supplied to it. Calculate (a) the change in internal energy of the gas (b) the number of moles in the gas if the initial temperature is 300 K (c) the molar heat capacity Cp at constant pressure and (d) the molar heat capacity Cv at constant volume.
A mixture contains 1 mole of helium (Cp = 2.5 R, Cv = 1.5 R) and 1 mole of hydrogen (Cp= 3.5 R, Cv = 2.5 R). Calculate the values of Cp, Cv and γ for the mixture.
Air (γ = 1.4) is pumped at 2 atm pressure in a motor tyre at 20°C. If the tyre suddenly bursts, what would be the temperature of the air coming out of the tyre? Neglect any mixing with the atmospheric air.
The speed of sound in hydrogen at 0°C is 1280 m s−1. The density of hydrogen at STP is 0.089 kg m−3. Calculate the molar heat capacities Cp and Cv of hydrogen.
4.0 g of helium occupies 22400 cm3 at STP. The specific heat capacity of helium at constant pressure is 5.0 cal K−1 mol−1. Calculate the speed of sound in helium at STP.
Molar specific heat of water is C = 74.7 J/mol K, its value in cal/g K is ______.
A diatomic molecule can be modelled as two rigid balls connected with spring such that the balls can vibrate with respect to centre of mass of the system (spring + balls). Consider a diatomic gas made of such diatomic molecule. If the gas performs 20 Joule of work under isobaric condition, then heat given to the gas is ______ J.
