Advertisements
Advertisements
Question
Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following:
\[\ce{[CoF6]^{3-}, [Co(H2O)6]^{2+}, [Co(Cn)6]^{3-}}\]
Solution
(1) \[\ce{[CoF6]^{3-}}\]:
\[\ce{Co^{3+} = 3d^6}\]
Number of unpaired electrons = 4
Magnetic moment = `sqrt(n(n + 2)) = sqrt(4(4 + 2))` = 4.9 BM
(ii) \[\ce{[Co(H2O)6]^{2+}}\]:

\[\ce{Co^{2+} = 3d^7}\]
Number of unpaired electrons = 3
Magnetic moment = `sqrt(3(3 + 2))` = 3.87 BM
(iii) \[\ce{[Co(CN)6]^{3-}}\]:
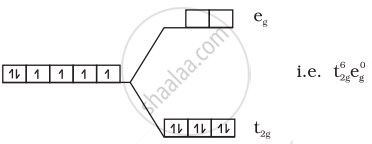
\[\ce{Co^{3+} = 3d^6}\]
No unpaired electrons so diamagnetic.
APPEARS IN
RELATED QUESTIONS
Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Why are low spin tetrahedral complexes rarely observed?
An aqueous pink solution of cobalt (II) chloride changes to deep blue on addition of excess of HCl. This is because:
(i) \[\ce{[Co(H2O)6]^{2+}}\] is transformed into \[\ce{[CoCl6]}^{4-}\]
(ii) \[\ce{[Co(H2O)6]^{2+}}\] is transformed into \[\ce{[CoCl4]}^{2-}\]
(iii) tetrahedral complexes have smaller crystal field splitting than octahedral complexes.
(iv) tetrahedral complexes have larger crystal field splitting than octahedral complex.
Arrange following complex ions in increasing order of crystal field splitting energy (∆O):
\[\ce{[Cr(Cl)6]^{3-}, [Cr(CN)6]^{3-}, [Cr(NH3)6]^{3+}}\].
Match the complex ions given in Column I with the hybridisation and number of unpaired electrons given in Column II and assign the correct code:
| Column I (Complex ion) | Column II (Hybridisation, number of unpaired electrons) |
| A. \[\ce{[Cr(H2O)6]^{3+}}\] | 1. dsp2, 1 |
| B. \[\ce{[Co(CN)4]^{2-}}\] | 2. sp3d2, 5 |
| C. \[\ce{[Ni(NH3)6]^{2+}}\] | 3. d2sp3, 3 |
| D. \[\ce{[MnF6]^{4-}}\] | 4. sp3, 4 |
| 5. sp3d2, 2 |
[Ni(H2O)6]2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+ (aq)is blue in colour, give reason in support of your answer.
Crystal field stabilising energy for high spind4 octahedral complex is:-
On the basis of Crystal Field theory, write the electronic configuration for the d5 ion with a strong field ligand for which Δ0 > P.
On the basis of crystal field theory, write the electronic configuration for the d5 ion with a weak ligand for which Δ0 < P.
Explain the difference between a weak field ligand and a strong field ligand.
