Advertisements
Advertisements
Question
Using de Broglie’s hypothesis, explain with the help of a suitable diagram, Bohr’s second postulate of quantization of energy levels in a hydrogen atom.
Solution
De Broglie’s Explanation of Bohr’s Second Postulate of Quantisation
• De-Broglie’s hypothesis that electron has a wavelength λ = h/mv gave an explanation for Bohr’s quantised orbits by bringing in the wave particle duality.
• Orbits correspond to circular standing waves in which the circumference of the orbits equal whole number of wavelength.
According to de Broglie’s hypothesis
`lambda = h/p`also for Bohr’s model
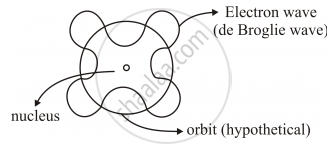
It states,
`nlambda = 2pir`
`=> n h/p = 2pir`
`=> rp =n h/(2pi) ` (as rp =L)
`=> L=n h/(2pi)` Bohr's 2ndPostulate
APPEARS IN
RELATED QUESTIONS
Calculate the de Broglie wavelength of an electron moving with - of the speed of light in vacuum (Negelct relativistic effect)
(Planck's constant: h = 6.63 x 10-34 Js, Mass of electron : m = 9.11 x 10-28 g)
An α-particle and a proton are accelerated through the same potential difference. Find the ratio of their de Broglie wavelength.
State de Broglie hypothesis
A deuteron and an alpha particle are accelerated with the same accelerating potential greater value of de-Broglie wavelength, associated it ?
What is meant by diffraction of light?
Answer the following question.
Obtain the expression for the ratio of the de-Broglie wavelengths associated with the electron orbiting in the second and third excited states of the hydrogen atom.
Light of wavelength 2000 Å falls on a metal surface of work function 4.2 eV.
What is the kinetic energy (in eV) of the fastest electrons emitted from the surface?
Light of wavelength 2000 Å falls on a metal surface of work function 4.2 eV.
What will be the change in the energy of the emitted electrons if the intensity of light with same wavelength is doubled?
A particle is dropped from a height H. The de Broglie wavelength of the particle as a function of height is proportional to
The work function of a metal is 4.50 eV. Find the frequency of light to be used to eject electrons from the metal surface with a maximum kinetic energy of 6.06 × 10−19 J.
