Advertisements
Advertisements
Questions
When you add sodium hydrogen carbonate to acetic acid in a test tube, a gas liberates immediately with brisk effervescence. Name this gas. Describe the method of testing this gas.
List two observations which you make when you add a pinch of sodium hydrogen carbonate to acetic acid in a test tube. Write chemical equation for the reaction that occurs.
Solution
Carbon dioxide gas gets liberated.
When a pinch of sodium hydrogen carbonate is added to acetic acid in a test tube, a brisk effervescence is produced because of the liberation of carbon dioxide gas.
When this gas is passed through the lime water, it turns lime water milky. This shows that the gas liberated is carbon dioxide gas.
The chemical reaction can be represented as
CH3COOH(aq) + NaHCO3(S) → CH3COONa(aq) + H2O(l) + CO2(g) ↑
APPEARS IN
RELATED QUESTIONS
A student takes about 2 mL ethanoic acid in a dry test tube and adds a pinch of sodium hydrogen carbonate to it. He reports the following observations:
I. Immediately a colourless and odourless gas evolves with a brisk effervescence.
II. The gas turns lime water milky when passed through it.
III. The gas burns with an explosion when a burning splinter is brought near it.
IV. The gas extinguishes the burning splinter that is brought near it.
The correct observations are
(A) I, II and III
(B) II, III and IV
(C) III, IV and I
(D) I, II and IV
Name the substance which on treatment with chlorine yields bleaching powder?
What will happen if a solution of sodium hydrocarbonate is heated? Give the equation of the reaction involved.
Give two important uses of baking soda.
Complete and balance the following chemical equations:
`NaHCO_3` 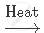
Describe how sodium hydrogencarbonate (baking soda) is produced on a large scale. Write equation of the reaction involved.
In addition to sodium hydrogencarbonate, baking powders contain a substance X. Name the substance X. What is the role of substance X in the baking powder?
Consider the following substances:
NaCl, Ca(OH)2, NaHCO3, NH3, Na2CO3, H2O, Cl2, CO2, CaSO4.2H2O, 2CaSO4.H2O, CaOCl2
Which compound is a part of baking powder?
Salt 'P', commonly used in bakery products, on heating gets converted into another salt 'Q' which itself is used for the removal of hardness of water and a gas 'R' is evolved. The gas 'R', when passed through freshly prepared lime water, turns milky. Identify 'P', 'Q' and 'R', giving a chemical equation for the justification of your answer.
Which one of the following salts does not contain water of crystallisation?
