Advertisements
Advertisements
Question
Write the approximate value of specific latent heat of ice.
Solution
The specific latent heat of ice is, 336000 J kg-1.
APPEARS IN
RELATED QUESTIONS
State two characteristics of a good thermion emitter.
State the effect of an increase of impurities on the melting point of ice.
State any two measures to minimize the impact of global warming.
- Which requires more heat: 1 g ice at 0℃ or 1 g water at 0℃ to raise its temperature to 10℃?
- Explain your answer in part (a).
A thermally insulated pot has 150 g ice at temperature 0°C. How much steam of 100°C has to be mixed to it, so that water of temperature 50°C will be obtained? (Given : latent heat of melting of ice = 80 cal/g, latent heat of vaporization of water = 540 cal/g, specific heat of water = 1 cal/g °C)
Explain the following temperature Vs. time graph:
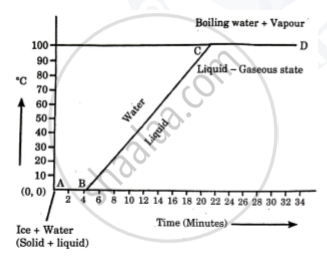
Define the following terms:
(i) Specific latent heat,
(ii) Specific latent heat of fusion.
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
Define specific latent heat of vaporization of a substance.
Explain why water is used in hot water bottles for fomentation and also as a universal coolant.
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
Explain the statement; “The specific latent heat of vaporization of wafer is 2260 × 103 J/kg”.
If pressure increases, the melting point of a substance ______.
Find the odd one out and give its explanation.
Write scientific reason.
The bottom of some steel utensils used for cooking is copper.
Calculate the total amount of heat energy required to melt 200 g of ice at 0°C to water at 100°C. (Specific latent heat of ice = 336 Jg-1, specific heat capacity of water = 4.2 Jg-1 °C-1)
The amount of heat energy required to melt a given mass of a substance at its melting point without any rise in its temperature is called as the ______.
20 g of ice at 0°C absorbs 10,920 J of heat energy to melt and change to water at 50°C. Calculate the specific latent heat of fusion of ice. Specific heat capacity of water is 4200 J kg-1 K-1.
