Advertisements
Online Mock Tests
Chapters
1: Force
2: Work, Energy and Power
3: Machines
LIGHT
4: Refraction of Light at Plane Surfaces
5: Refraction through a Lens
6: Spectrum
SOUND
7: Sound
ELECTRICITY AND MAGNETISM
8: Current Electricity
9: Household Circuits
10: Electro-Magnetism
HEAT
▶ 11: Calorimetry
MODERN PHYSICS
12: Radioactivity
![Selina solutions for Physics [English] Class 10 ICSE chapter 11 - Calorimetry Selina solutions for Physics [English] Class 10 ICSE chapter 11 - Calorimetry - Shaalaa.com](/images/physics-english-class-10-icse_6:4c973dd038c545c9a2b6db170ad2f542.jpg)
Advertisements
Solutions for Chapter 11: Calorimetry
Below listed, you can find solutions for Chapter 11 of CISCE Selina for Physics [English] Class 10 ICSE.
Selina solutions for Physics [English] Class 10 ICSE 11 Calorimetry EXERCISE-11(A) [Pages 269 - 271]
Define the term heat.
Name the SI unit of heat.
Define the term calorie. How is it related to joule (the S.I. unit of heat)?
Define one kilo-calorie of heat.
Define temperature.
Write S.I. unit of temperature.
State three differences between heat and temperature.
What is the principle of Calorimetry?
Define the term heat capacity.
State S.I. unit of heat capacity.
Define specific heat capacity.
State S.I. unit of specific heat capacity.
How is heat capacity of a body related to specific heat capacity of its substance?
State two differences between "Heat Capacity" and "Specific Heat Capacity".
Name a liquid which has the highest specific heat capacity.
What do you mean by the following statement?
The heat capacity of a body is 50 JK-1?
What do you mean by the following statement?
The specific heat capacity of copper is 0. 4 Jg-1 K-1?
Specific heat capacity of a substance A is 3.8 J g-1 K-1 and of substance B is 0.4 J g-1 k-1. Which substance is a good conductor of heat? How did you arrive at your conclusion?
Name two factors on which the heat energy librated by a body on cooling depends.
Name three factors on which heat energy absorbed by a body depends and state how does it depend on them.
Write the expression for the heat energy Q received by the substance when m kg of substance of specific heat capacity c Jkg-1 k-1 is heated through Δt° C.
Same amount of heat is supplied to two liquid A and B. The liquid A shows a greater rise in temperature. What can you say about the heat capacity of A as compared to that of B?
Two blocks P and Q of different metals having their mass in the ratio 2 : 1 are given same amount of heat. Their temperature rises by same amount. Compare their specific heat capacities.
What is the principle of the method of mixtures?
What is the other name given to the principle of the mixtures?
Name the law on which the principle of mixture is based.
A mass m1 of a substance of specific heat capacity c1 at temperature t1 is mixed with a mass m2 of other substance of specific heat capacity c2 at a lower temperature t2. Deduce the expression for the temperature t of the mixture. State the assumption made, if any.
Discuss the role of high specific heat capacity of water with reference to climate in coastal areas.
Water is used in hot water bottles for fomentation. Give a reason.
What property of water makes it an effective coolant?
Give one example where high specific heat capacity of water is used as cooling.
Give one example where high specific heat capacity of water is used as heat reservoir.
A liquid X has specific heat capacity higher than the liquid Y. Which liquid is useful as coolant in car radiators.
A liquid X has specific heat capacity higher than the liquid Y. Which liquid is useful as heat reservoir to keep juice bottles without freezing?
What is a calorimeter?
Name the material of which it is made of. Give two reasons for using the material stated by you.
Out of the three metals A, B and C of specific heat 900 J kg-1 °C-1, 380 J kg-1 °C-1 and 460 J kg-1 °C-1 respectively, which will you prefer for calorimeter? Given reason.
How is the loss of heat due to radiation minimised in a calorimeter?
Why is the base of a cooking pan generally made thick?
MULTIPLE CHOICE TYPE
The S.I. unit of heat capacity is ______.
J kg-1
J K-1
J kg-1 K-1
cal °C-1
The S.I. unit of specific heat capacity is ______.
J kg-1
J K-1
J kg-1 K-1
kilocal kg-10C-1
The specific heat capacity of water is ______.
4200 Jkg-1K-1
420 Jg-1K-1
0.42 Jg-1K-1
4.2 Jkg-1K-1
NUMERICALS
By imparting heat to a body its temperature rises by 15°C. What is the corresponding rise in temperature on kelvin scale?
- Calculate the heat capacity of a copper vessel of mass 200 g if the specific heat capacity of copper is 410 J kg-1 K-1.
- How much heat energy will be required to increase the temperature of the vessel in part (a) from 25°C to 35°C?
A piece of iron of mass 2.0 kg has a heat capacity of 966 J K-1. Find heat energy needed to warm it by 15°C.
A piece of iron of mass 2.0 kg has a heat capacity of 966 J K-1. Find its specific heat capacity in S.I unit.
Calculate the amount of heat energy required to raise the temperature of 200 g of copper from 20°C to 70°C. Specific heat capacity of copper = 390 J kg-1 K-1.
1300 J of heat energy is supplied to raise the temperature of 6.5 kg of lead from 20° C to 40°C. Calculate the specific heat capacity of lead.
Find the time taken by a 500 W heater to raise the temperature of 50 kg of material of specific heat capacity 960 J kg-1K-1, from 18°C to 38° C. Assume that all the heat energy supplied by the heater is given to the material.
An electric heater of power 600 W raises the temperature of 4.0 kg of a liquid from 10.0℃ to 15.0℃ in100 s. Calculate:
- the heat capacity of 4.0 kg of liquid,
- the specific heat capacity of the liquid.
0.5 kg of lemon squash at 30° C is placed in a refrigerator which can remove heat at an average rate of 30 J s−1. How long will it take to cool the lemon squash to 5°C? Specific heat capacity of squash = 4200 J g−1K−1.
A mass of 50 g of a certain metal at 150° C is immersed in 100 g of water at 11° C. The final temperature is 20° C. Calculate the specific heat capacity of the metal. Assume that the specific heat capacity of water is 4.2 J g-1 K-1.
45 g of water at 50°C in a beaker is cooled when 50 g of copper at 18° C is added to it. The contents are stirred till a final constant temperature is reached. Calculate this final temperature. The specific heat capacity of copper is 0.39 J g-1K-1 and that of water is 4.2 J g-1K-1. State the assumption used.
200 g of hot water at 80°C is added to 400 g of cold water at 10°C. Neglecting the heat taken by the container, calculate the final temperature of the mixture of water. Specific heat capacity of water = 4200 J kg-1K-1.
1.0 kg of water is contained in a 1.25 kW kettle. Calculate the time taken for the temperature of water to rise from 25° C to its boiling point of 100°C. Specific heat capacity of water = 4.2 J g-1K-1.
Selina solutions for Physics [English] Class 10 ICSE 11 Calorimetry EXERCISE-11 (B) [Pages 280 - 282]
What do you understand by the change of phase of a substance?
Is there any change in temperature during the change of phase?
Does the substance absorb or liberate any heat energy during the change of phase?
What is the name given to the energy absorbed during a phase change?
A substance changes from its solid state to the liquid state when heat is supplied to it. Name the process.
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
A substance changes from its solid state to the liquid state when heat is supplied to it. How does the average kinetic energy of the molecules of the substance change?
A substance on heating, undergoes
- a change in its temperature,
- a change in its phase without change in its temperature.
In each case, state the change in energy of molecules of the substance.
How does the average kinetic energy of molecules of a substance change during its change in phase at a constant temperature on heating?
How does the average potential energy of molecules of a substance change during its change in phase at a constant temperature on heating?
State the effect of presence of impurity on the melting point of ice. Give one use of it.
State the effect of increase of pressure on the melting point of ice.
The diagram in Figure below shows the change of phase of a substance on a temperature time graph on heating the substances at a constant rate.
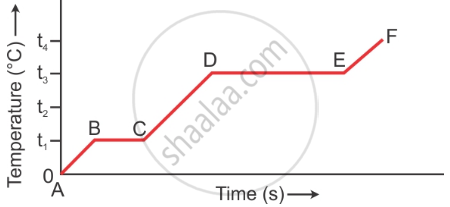
- What do the parts AB, BC, CD and DE represent?
- What is the melting points of the substance?
- What is the boiling points of the substance?
The melting point of napthalene is 80°C and the room temperature is 25°. A sample of liquid napthalene at 90° is cooled down to room temperature. Draw a temperature-time graph to represent this cooling. On the graph mark the region which corresponds to the freezing process.
1 kg of ice at 0°C is heated at a constant rate and its temperature is recorded after every 30 s till steam is formed at 100°C. Draw a temperature-time graph to represent the change of phases.
Explain the terms boiling.
Explain the terms boiling point.
How is the volume of water affected when it boils at 100℃?
How is the boiling point of water affected when some salt is added to it?
What is the effect of increase in pressure on the boiling point of a liquid?
Water boils at 120°C in a pressure cooker. Explain the reason.
Write down the approximate range of temperature at which water boils in a pressure cooker.
It is difficult to cook vegetables on hills and mountains. Explain the reason.
Complete the following sentences:
When ice melts, its volume ______.
Decrease in pressure over ice ______ its melting point.
Increase in pressure ______ the boiling point of water.
A pressure cooker is based on the principle that boiling point of water increases with the ______.
The boiling point of water is defined as ______.
Water can be made to boil at 115°C by ______ pressure over its surface.
What do you understand by the term latent heat?
Define the term specific latent heat of fusion of ice. State its S.I. unit.
Write the approximate value of specific latent heat of ice.
The specific latent heat of fusion of ice is 336 J g-1. Explain the meaning of its statement.
1 g ice of 0℃ melts to form 1 g water at 0℃. State whether the latent heat is absorbed or given out by ice.
Which has more heat: 1 g ice at 0℃ or 1g water 0℃? Give reason.
- Which requires more heat: 1 g ice at 0℃ or 1 g water at 0℃ to raise its temperature to 10℃?
- Explain your answer in part (a).
Ice cream appears colder to the mouth than water at 0℃. Give reason.
The soft drink bottles are cooled by
- ice cubes at 0°C
- iced-water at 0°C.
Which will cool the drink quickly? Give reason.
It is generally cold after a hail-storm then during and before the hail storm. Give reason.
The temperature of the surrounding starts falling when ice in a frozen lake starts melting. Give reason.
Water in lakes and ponds do not freeze at once in cold countries. Give reason.
Explain the following:
The surrounding become pleasantly warm when water in a lake starts freezing in cold countries.
Explain the following:
The heat supplied to a substance during it change of state, does not cause any rise in its temperature.
MULTIPLE CHOICE TYPE
The S.I. unit of specific latent heat is ______.
cal g-1
cal g-1 K-1
J kg-1
J kg -1 K-1
The specific latent heat of fusion of water is ______.
80 cal g-1
2260 J g-1
80 J g-1
336 J kg-1
NUMERICALS
20 g of ice at 0°C absorbs 10,920 J of heat energy to melt and change to water at 50°C. Calculate the specific latent heat of fusion of ice. Specific heat capacity of water is 4200 J kg-1 K-1.
How much heat energy is released when 5.0 g of water at 20℃ changes into ice at 0℃? Take specific heat capacity of water = 4.2 J g-1 K-1, Specific latent heat of fusion of ice = 336 J g-1.
A molten metal of mass 150 g is kept at its melting point 800℃. When it is allowed to freeze at the same temperature, it gives out 75,000 J of heat energy.
- What is the specific latent heat of the metal?
- If the specific heat capacity of metal is 200 J kg-1 K-1, how much additional heat energy will the metal give out in cooling to -50℃?
A solid metal weighing 150 g melts at its melting point of 800 °C by providing heat at the rate of 100 W. The time taken for it to completely melt at the same temperature is 4 min. What is the specific latent heat of fusion of the metal?
A refrigerator converts 100g of water at 20℃ to ice at – 10℃ in 73.5 min. Calculate the average rate of heat extraction in watt. The specific heat capacity of water is 4.2 J kg-1 K-1, specific latent heat of ice is 336 J g-1 and the specific heat capacity of ice is 2.1 J kg-1 K-1.
In an experiment, 17g of ice is used to bring down the temperature of 40 g of water at 34℃ to its freezing temperature. The specific heat capacity of water is 4.2 J g-1K-1. Calculate the specific latent heat of ice. State one important assumption made in the above calculation.
The temperature of 170 g of water at 50°C is lowered to 5°C by adding a certain amount of ice to it. Find the mass of ice added.
Given: Specific heat capacity of water = 4200 J kg-1 °C-1 and specific latent heat of ice = 336000 J kg-1.
Find the result of mixing 10 g of ice at - 10℃ with 10 g of water at 10℃. Specific heat capacity of ice = 2.1 J kg-1 K-1, Specific latent heat of ice = 336 J g-1 and specific heat capacity of water = 4.2 J kg-1 K-1.
A piece of ice of mass 40 g is added to 200 g of water at 50 °C. Calculate the final temperature of water when all the ice has melted. Specific heat capacity of water = 4200 J kg-1K-1, and specific latent heat of fusion of ice = 336 × 103 J kg-1.
Calculate the mass of ice needed to cool 150 g of water contained in a calorimeter of mass 50 g at 32 °C such that the final temperature is 5 °C. Specific heat capacity of calorimeter = 0.4 J g-1 °C-1, Specific heat capacity of water = 4.2 J g-1°C-1, latent heat capacity of ice = 330 J g-1.
250 g of water at 30℃ is contained in a copper vessel of mass 50 g. Calculate the mass of ice required to bring down the temperature of the vessel and its contents to 5℃. Given: specific latent heat of fusion of ice = 336 × 103J kg-1, specific heat capacity of copper = 400 J kg-1 K-1, specific heat capacity of water = 4200 J kg-1 K-1.
2 kg of ice melts when water at 100℃ is poured in a hole drilled in a block of ice. What mass of water was used? Given: specific heat capacity of water = 4200 J kg-1 K-1, specific latent heat of ice = 336 × 103 J kg-1.
Calculate the total amount of heat energy required to convert 100 g of ice at −10℃ completely into water at 100℃. Specific heat capacity of ice = 2.1 J g-1 K-1, specific heat capacity of water = 4.2 J g-1K-1, specific latent heat of ice = 336 J g-1.
The amount of heat energy required to convert 1 kg of ice at – 10℃ to water at 100℃ is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity of ice = 2100 J kg-1K-1, Specific heat capacity of water = 4200 J kg-1 K-1.
200 g of ice at 0 °C converts into water at 0°C in 1 minute when heat is supplied to it at a constant rate. In how much time, 200 g of water at 0°C will change to 20°C ? Take specific latent heat of ice = 336 J g-1.
Solutions for 11: Calorimetry
![Selina solutions for Physics [English] Class 10 ICSE chapter 11 - Calorimetry Selina solutions for Physics [English] Class 10 ICSE chapter 11 - Calorimetry - Shaalaa.com](/images/physics-english-class-10-icse_6:4c973dd038c545c9a2b6db170ad2f542.jpg)
Selina solutions for Physics [English] Class 10 ICSE chapter 11 - Calorimetry
Shaalaa.com has the CISCE Mathematics Physics [English] Class 10 ICSE CISCE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Selina solutions for Mathematics Physics [English] Class 10 ICSE CISCE 11 (Calorimetry) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Selina textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Physics [English] Class 10 ICSE chapter 11 Calorimetry are Heat and Its Unit, The Temperature and a Thermometer, Thermal Capacity (Heat Capacity), Specific Heat Capacity, Relationship Between the Heat Capacity and Specfic Heat Capacity, Calorimetry and Calorimeter, Natural Phenomena and Consequences of High Specific Heat Capacity of Water, Some Examples of High and Low Heat Capacity, Heat and change of physical state, Factors Affecting the Quantity of Heat Absorbed to Increase the Temperature of a Body, Difference Between Heat and Temperature, Effect of Pressure on the Melting Point, Concept of Boiling (Vaporization), Effect of Impurities on the Melting Point, Change in Volume on Boiling, Effect of Pressure on the Boiling Point, Effect of Impurities on the Boiling Point, Melting and Freezing, Heating Curve of Ice During Melting, Change in Volume on Melting, Heating Curve for Water, Explanation of Latent Heat of Melting on the Basis of Kinetic Model, Specific Heat Capacity of Some Common Substances, Latent Heat and Specific Latent Heat, Natural Consequences of High Specific Latent Heat of Fusion of Ice, Principle of Method of Mixtures (or Principle of Calorimetry), Latent Heat and Specific Latent Heat, Heat and Its Unit, The Temperature and a Thermometer, Thermal Capacity (Heat Capacity), Specific Heat Capacity, Relationship Between the Heat Capacity and Specfic Heat Capacity, Calorimetry and Calorimeter, Natural Phenomena and Consequences of High Specific Heat Capacity of Water, Some Examples of High and Low Heat Capacity, Heat and change of physical state, Factors Affecting the Quantity of Heat Absorbed to Increase the Temperature of a Body, Difference Between Heat and Temperature, Effect of Pressure on the Melting Point, Concept of Boiling (Vaporization), Effect of Impurities on the Melting Point, Change in Volume on Boiling, Effect of Pressure on the Boiling Point, Effect of Impurities on the Boiling Point, Melting and Freezing, Heating Curve of Ice During Melting, Change in Volume on Melting, Heating Curve for Water, Explanation of Latent Heat of Melting on the Basis of Kinetic Model, Specific Heat Capacity of Some Common Substances, Latent Heat and Specific Latent Heat, Natural Consequences of High Specific Latent Heat of Fusion of Ice, Principle of Method of Mixtures (or Principle of Calorimetry), Latent Heat and Specific Latent Heat.
Using Selina Physics [English] Class 10 ICSE solutions Calorimetry exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Selina Solutions are essential questions that can be asked in the final exam. Maximum CISCE Physics [English] Class 10 ICSE students prefer Selina Textbook Solutions to score more in exams.
Get the free view of Chapter 11, Calorimetry Physics [English] Class 10 ICSE additional questions for Mathematics Physics [English] Class 10 ICSE CISCE, and you can use Shaalaa.com to keep it handy for your exam preparation.
