Advertisements
Advertisements
Question
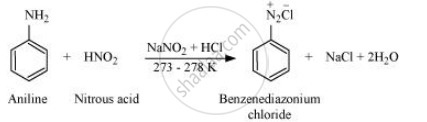
Write the reaction of aromatic primary amine with nitrous acid.
Solution
Aromatic amine react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at 273 − 278 K to form stable aromatic diazonium salts, i.e., NaCl and H2O.
APPEARS IN
RELATED QUESTIONS
Identify the compounds 'A' and 'B' in the following equation:

An aromatic compound 'A' of molecular formula C7H7ON undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions :

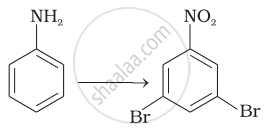
Accomplish the following conversion:
Nitrobenzene to benzoic acid
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
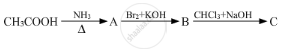
Give the structures of A, B and C in the following reactions :
Account for the following:
Gabriel phthalimide synthesis is not preferred for preparing aromatic primary amines.
The following amines is the product of Gabriel phthalimide synthesis.
Write the order of reactivity of alkyl halides with ammonia.
Write reactions to bring about the following conversions.
Acetamide to Ethylamine
Identify the INCORRECT statement regarding Hofmann bromamide reaction.
In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one \[\ce{CH2}\] group in the carbon chain, the reagent used as source of nitrogen is ______.
Hoffmann Bromamide Degradation reaction is shown by ______.
What is the best reagent to convert nitrile to primary amine?
How will you carry out the following conversions?
The compound X is which of the following?
\[\ce{CH3CN ->[Na + C2H5OH] x}\]
Ethylamine can be prepared by the action of bromine and caustic potash on which compound?
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
Which of the following amines can be prepared by Gabriel phthalimide reaction?
Write short notes on the following:
Ammonolysis
