Advertisements
Advertisements
Question
Write the structures of two compounds having molecular formula C3H6O and give their names.
Solution
The isomers of C3H6O include:
propanal
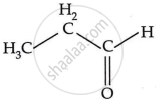
propanone
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{O}\phantom{...}\ce{H}\\
|\phantom{....}||\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.........}|\\
\ce{H}\phantom{........}\ce{H}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential condition for an addition reaction. Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction.
Write the number of covalent bonds in the molecule of propane, C3H8.
What is meant by isomers?
Explain why propane cannot exhibit the structural isomerism property.
Give the name and structural formula of one member each of the following:
alkyne
How many isomers of the following hydrocharbons are possible?
C4H10
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if B is an open chain compound
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae can represent cyclic hydrocarbons?
What is the molecular formula and structural formula of a cyclic hydrocarbon whose one molecule contains 8 hydrogen atoms?
Draw two possible isomers of the compound with molecular formula C3H6O and write their names.
Give the electron dot structures of the above two compounds.
