English Medium
Academic Year: 2022-2023
Date & Time: 4th March 2023, 10:30 am
Duration: 3h
Advertisements
General Instructions:
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. Students are expected to attempt only one of these questions.
- Section A consists of 20 objective type questions carrying 1 mark each. Q. No. 17 to 20 are Assertion - Reasoning based questions.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should be in the range of 50 to 80 words
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
- There is no overall choice. However, an internal choice has been provided in some Sections.
When sodium bicarbonate reacts with dilute hydrochloric acid, the gas evolved is ______.
Hydrogen; it given pop sound with burning match stick.
Hydrogen; it turns lime water milky.
Carbon dioxide; it turns lime water milky.
Carbon dioxide; it blows off a burning match stick with a pop sound.
Chapter:
When aqueous solutions of potassium iodide and lead nitrate are mixed an insoluble substance separates out. The chemical equation for the reaction involved is:
\[\ce{KI + PbNO_3 ->PbI + KNO3}\]
\[\ce{2KI + Pb(NO_3)2 ->PbI2 + 2KNO3}\]
\[\ce{KI + Pb(NO_3)2 ->PbI + KNO3}\]
\[\ce{KI + Pb(NO_3)2 ->PbI2 + 2KNO3}\]
Chapter: [0.01] Chemical Reactions and Equations
A metal ribbon 'X' bums in oxygen with a dazzling white flame forming a white ash 'Y'. The correct description of X, Y and the type of reaction is:
X = Ca; Y = CaO;
Type of reaction = Decomposition
X = Mg; Y = MgO;
Type of reaction = Combination
X = Al; Y = Al2O3;
Type of reaction = Thermal decomposition
X = Zn; Y = ZnO;
Type of reaction = Endothermic
Chapter: [0.01] Chemical Reactions and Equations
Acid present in tomato is ______.
Methanoic acid
Acetic acid
Lactic acid
Oxalic acid
Chapter: [0.02] Acids, Bases and Salts
Sodium hydroxide is termed an alkali while Ferric hydroxide is not because ______.
Sodium hydroxide is a strong base, while Ferric hydroxide is a weak base.
Sodium hydroxide is a base which is soluble in water while Ferric hydroxide is also a base but it is not soluble in water.
Sodium hydroxide is a strong base while Ferric hydroxide is a strong acid.
Sodium hydroxide and Ferric hydroxide both are strong base but the solubility of sodium hydroxide in water is comparatively higher than that of Ferric hydroxide.
Chapter: [0.02] Acids, Bases and Salts
The name of the salt used to remove permanent hardness of water is ______.
Sodium hydrogen carbonate (NaHCO3)
Sodium chloride (NaCl)
Sodium carbonate decahydrate (Na2CO3.10H2O)
Calcium sulphate hemihydrate (CaSO4.`1/2`H2O)
Chapter: [0.02] Acids, Bases and Salts
The electron dot structure of chlorine molecule is:

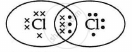
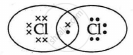

Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
Observe the following diagram and identify the process and its significance from the following options:

Evaporation: maintains water contents in leaf cells.
Transpiration: creates a suction force which pulls water inside the plant.
Excretion: helps in excreting out waste water from the plant.
Translocation: helps in transporting materials from one cell to another.
Chapter: [0.05] Life Processes
During adolescence, reproductive phase starts and ______.
general growth rate begin to show down
height becomes less
the body weight is reduced
hair growth decreases
Chapter: [0.07] How do Organisms Reproduce?
Which pair of sex chromosome will determine a male?
XO
XX
XY
YY
Chapter: [0.08] Heredity
One of the events that does not occur during photosynthesis is ______.
Chlorophyll absorbs solar energy.
Carbon dioxide is released during the process.
Oxygen is released during the process.
Carbon dioxide is absorbed during the process.
Chapter: [0.02] Acids, Bases and Salts
The number of chromosomes in parents and offsprings of a particular species undergoing sexual reproduction remain constant due to:
doubling of chromosomes after zygote formation.
the halving of chromosomes during gamete formation.
doubling of chromosomes after gamete formation.
the halving of chromosomes after gamete formation.
Chapter: [0.07] How do Organisms Reproduce?
Two LED bulbs of 12W and 6W are connected in series. If the current through 12W bulb is 0.06A the current through 6W bulb will be ______.
0.04 A
0.06 A
0.08 A
0.12 A
Chapter: [0.12] Magnetic Effects of Electric Current
The correct pattern of magnetic field lines of the field produced by a current carrying circular loop is:



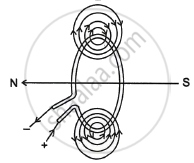
Chapter: [0.12] Magnetic Effects of Electric Current
The resistance of a resistor is reduced to half of its initial value. If other parameters of the electrical circuit remain unaltered, the amount of heat produced in the resistor will become ______.
four times
two times
half
one forth
Chapter: [0.11] Electricity
Two LED bulbs of 10W and 5W are connected in series. If the current flowing through 5W bulb is 0.005A, the current flowing through 10W bulb is ______.
0.02 A
0.01 A
0.005 A
0.0025 A
Chapter: [0.12] Magnetic Effects of Electric Current
Assertion (A): Reaction of Quicklime with water is an exothermic reaction.
Reason (R): Quicklime reacts vigorously with water releasing a large amount of heat.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.02] Acids, Bases and Salts
Assertion (A): In humans, if gene (B) is responsible for black eyes and gene (b) responsible for brown eyes, then the colour of eyes of the progeny having gene combination Bb, bb or BB will be black only.
Reason (R): The black colour of the eyes is a dominant trait.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.08] Heredity
Assertion (A): The inner walls of the small intestine have finger like projections called villi which are rich in blood.
Reason (R): These villi have large surface area to help the small intestine in completing the digestion of food.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.05] Life Processes
Assertion (A): A current carrying straight conductor experiences a force when placed perpendicular to the direction of magnetic field.
Reason (R): The net charge on a current carrying conductor is always zero.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.12] Magnetic Effects of Electric Current
State the essential function performed by ozone at the higher levels of the atmosphere.
Chapter: [0.13] Our Environment
Why was there a sharp in the amount of ozone in the atmosphere in 1980's.
Chapter: [0.13] Our Environment
Name the part of brain which is responsible for following actions:
- Maintaining posture and balance
- Beating of heart
- Thinking
- Blood pressure
Chapter: [0.06] Control and Co-ordination [0.06] Control and Co-ordination
Advertisements
Where are auxins synthesized in a plant? Which organ of the plant shows:
- Positive phototropism
- Negative geotropism
- Positive hydrotropism
Chapter: [0.06] Control and Co-ordination
Write one specific function each of the following organs in relation with excretion in human beings:
- Renal Artery
- Urethra
- Glomerulus
- Tubular part of nephron
Chapter: [0.05] Life Processes
Give two reasons, why bile juice is considered to be an important secretion of liver in the process of digestion?
Chapter: [0.05] Life Processes
Name the hormone secreted in scary situations by animals. Write any three responses which enable the animal body to deal with it.
Chapter: [0.06] Control and Co-ordination
Use of several pesticides which results in excessive accumulation of pesticides in rivers of ponds, is a matter of deep concern. Justify this statement.
Chapter: [0.13] Our Environment
While electrolysing water before passing the current some drops of an acid are added. Why? Name the gases liberated at cathode and anode. Write the relationship between the volume of gas collected at anode and the volume of gas collected at cathode.
Chapter: [0.02] Acids, Bases and Salts
What is observed when silver chloride is exposed to sunlight? Give the type of reaction involved.
Chapter: [0.01] Chemical Reactions and Equations
Suggest a safe procedure of diluting a strong concentrated acid.
Chapter: [0.02] Acids, Bases and Salts
Name the salt formed when sulphuric acid is added to sodium hydroxide and write its pH.
Chapter: [0.02] Acids, Bases and Salts
Dry HCl gas does not change the colour of dry blue litmus paper. Why?
Chapter: [0.02] Acids, Bases and Salts
List two difference in the characteristic properties of the virtual images formed by the two types of spherical lenses (concave and convex). How are these characteristics of the two lenses use in the correction of the two common defects of vision namely myopia and hypermetropia?
Chapter: [0.1] The Human Eye and the Colourful World
An object is kept at a distance of 1m from a lens of power +2D:
- Identify the type of lens.
- Calculate its focal length and distance of the image formed.
Chapter: [0.09] Light - Reflection and Refraction
Define the following terms in the context of a diverging mirror:
- Principal focus
- Focal length
Draw a labelled ray diagram to illustrate your answer.
Chapter: [0.09] Light - Reflection and Refraction
The power of a lens is +4D. Find the focal length of this lens. An object is placed at a distance of 50 cm from the optical centre of this lens. State the nature and magnification of the image formed by the lens and also draw a ray diagram to justify your answer.
Chapter: [0.09] Light - Reflection and Refraction
Why is acidified water considered to be a good conductor of electricity?
Chapter: [0.03] Metals and Non Metals
Write a chemical equation showing the ionic products formed on dissolving potassium hydroxide in water.
Chapter: [0.03] Metals and Non Metals
Care must be taken while diluting concentrated nitric acid with water. Why?
Chapter: [0.03] Metals and Non Metals
Write one difference between biodegradable and non-biodegradable wastes. List two impacts of each type of the accumulated waste on environment if not disposed off properly.
Chapter: [0.13] Our Environment
Advertisements
Draw the structure of butanoic acid.
Chapter: [0.04] Carbon and its Compounds
Draw the structure of the following compound:
Chloropentane
Chapter:
How are structure (i) and structure (ii) given below related to one another? Give reason to justify your answer.
Structure (i) \[\begin{array}{cc} \ce{CH3}\phantom{............}\ce{CH3}\\ \backslash\phantom{..........}/\\ \ce{CH - CH}\\ /\phantom{..........}\backslash\\ \ce{CH3}\phantom{............}\ce{CH3} \end{array}\]
Structure (ii) \[\begin{array}{cc} \ce{CH3}\phantom{...........}\\ \backslash\phantom{.......}\\ \ce{CH3 - C - CH2CH3}\\ /\phantom{.......}\\ \ce{CH3}\phantom{...........} \end{array}\]
Chapter:
Differentiate between saturated and unsaturated carbon compounds on the basis of their general formula.
Chapter:
What happens when a small piece of sodium is dropped in ethanol? Write the equation for this reactions.
Chapter: [0.04] Carbon and its Compounds
Why is glacial acetic acid called so?
Chapter: [0.04] Carbon and its Compounds
What happens when ethanol is heated at 443K in the presence of conc. H2SO4? Write the role of conc. H2SO4 in this case.
Chapter: [0.04] Carbon and its Compounds
Write an equation showing saponification.
Chapter: [0.04] Carbon and its Compounds
Name and explain the two modes of asexual reproduction observed in hydra.
Chapter: [0.07] How do Organisms Reproduce?
What is vegetative propagation?
Chapter: [0.07] How do Organisms Reproduce?
List two advantages of vegetative propagation technique.
Chapter: [0.07] How do Organisms Reproduce?
It is observed that covalent compounds are had conductors of electricity. Give reason.
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
Carbon can neither form C4- cation nor C4 anion. Why?
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
Draw electron dot structure of Ethanol.
Chapter: [0.04] Carbon and its Compounds
Identify heteroatom (s) in the following compound.
\[\begin{array}{cc}
\ce{CH3CH2 - C - CH3}\\\
\phantom{....}||\
\ce\\\phantom{....}{O}\end{array}\]
Chapter: [0.04] Carbon and its Compounds
Identify heteroatom (s) in the following compound.
\[\ce{CH3CH2Cl}\]
Chapter: [0.04] Carbon and its Compounds
What are soaps?
Chapter: [0.04] Carbon and its Compounds
Explain the mechanism of cleansing action of soap with the help of a labelled diagram.
Chapter: [0.04] Carbon and its Compounds
Detergents are better than soaps. Justify.
Chapter: [0.04] Carbon and its Compounds
The melting points and boiling points of some ionic compounds are given below:
| Compound | Melting Point (K) |
Boiling Point (K) |
| NaCl |
1074 |
1686 |
| LiCl | 887 | 1600 |
| CaCl2 | 1045 | 1900 |
| CaO | 2850 | 3120 |
| MgCl2 | 981 | 1685 |
These compounds are termed ionic because they are formed by the transfer of electrons from a metal to a non-metal. The electron transfer in such compounds is controlled by the electronic configuration of the elements involved. Every element tends to attain a completely filled valence shell of its nearest noble gas or a stable octet.
- Show the electron transfer in the formation of magnesium chloride.
- List two properties of ionic compounds other than their high melting and boiling points.
- (A) While forming an ionic compound say sodium chloride how does sodium atom attain its stable configuration?
OR
(B) Give reasons:
(i) Why do ionic compounds in the solid state not conduct electricity?
(ii) What happens at the cathode when electricity is passed through an aqueous solution of sodium chloride?
Chapter:
| The most obvious outcome of the reproduction process is the generation of individuals of similar design, but in sexual reproduction they may not be exactly alike. The resemblances as well as differences are marked. The rules of heredity determine the process by which traits and characteristics are reliably inherited. Many experiments have been done to study the rules of inheritance. |
- Why an offspring of human being is not a true copy of his parents in sexual reproduction?
- While performing experiments on inheritance in plants, what is the difference between F1 and F2 generation?
- (A) Why do we say that variations are useful for the survival of a species over time?
OR
(B) Study Mendel's cross between two plants with a pair of contrasting characters.
RRYY × rryy Round Yellow Wrinkled Green He observed 4 types of combinations in F2 generation. Which of these were new combinations? Why do new features which are not present in the parents, appear in F2 generation?
Chapter: [0.07] How do Organisms Reproduce?
| The ability of medium to refract light is expressed in terms of its optical density. Optical density has a definite connotation. It is not the same as mass density. On comparing two media, the one with the large refractive index is optically denser medium than the other. The other medium with a lower refractive index is optically rarer. Also the speed of light through a given medium is inversely proportional to its optical density. |
- Determine the speed of light in diamond if the refractive index of diamond with respect to vacuum is 2.42. Speed of light in vacuum is 3 × 108 m/s.
- Refractive indices of glass, water and carbon disulphide are 1.5, 1.33 and 1.62 respectively. If a ray of light is incident in these media at the same angle (say θ), then write the increasing order of the angle of refraction in these media.
- (A) The speed of light in glass is 2 × 108 m/s and is water is 2.25 × 108 m/s.
(a) Which one of the two optically denser and why?
(b) A ray of light is incident normally at the water glass interface when it enters a thick glass container filled with water. What will happen to the path of the ray after entering the glass? Give reason.
OR
(B) The absolute refractive indices of glass and water are 4/3 and 3/2, respectively. If the speed of light in glass is 2 × 108 m/s, calculate the speed of light in (i) vacuum (ii) water.
Chapter: [0.09] Light - Reflection and Refraction
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2022 - 2023
Previous year Question paper for CBSE Class 10 Science-2023 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
