Advertisements
Advertisements
प्रश्न
An ideal gas is kept in a long cylindrical vessel fitted with a frictionless piston of cross-sectional area 10 cm2 and weight 1 kg. The length of the gas column in the vessel is 20 cm. The atmospheric pressure is 100 kPa. The vessel is now taken into a spaceship revolving round the earth as a satellite. The air pressure in the spaceship is maintained at 100 kPa. Find the length of the gas column in the cylinder.
Use R = 8.3 J K-1 mol-1
उत्तर
P1V1 = P2V2
⇒ `((mg)/A + P_0)Al = P_0Al``
⇒ `((1 xx 98)/(10 xx 10^-4) + 10^5)0.2 = 10^5 I``
⇒ (9.8 × 103 + 105) × 0.2 = 105I`
= 109.8 × 103 × 0.2 = 105I`
⇒ I' = `(109.8 xx 0.2)10^2`
= 0.2196 m
= 0.22 m ≈ 22 cm
APPEARS IN
संबंधित प्रश्न
From a certain apparatus, the diffusion rate of hydrogen has an average value of 28.7 cm3 s–1. The diffusion of another gas under the same conditions is measured to have an average rate of 7.2 cm3 s–1. Identify the gas
[Hint: Use Graham’s law of diffusion: R1/R2 = (M2/M1)1/2, where R1, R2 are diffusion rates of gases 1 and 2, and M1 and M2 their respective molecular masses. The law is a simple consequence of kinetic theory.]
While gas from a cooking gas cylinder is used, the pressure does not fall appreciably till the last few minutes. Why?
Explain why cooking is faster in a pressure cooker.
A gas behaves more closely as an ideal gas at
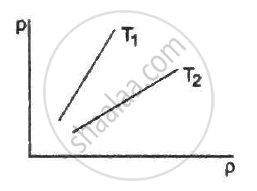
Figure shows graphs of pressure vs density for an ideal gas at two temperatures T1 and T2.
Equal masses of air are sealed in two vessels, one of volume V0 and the other of volume 2V0. If the first vessel is maintained at a temperature 300 K and the other at 600 K, find the ratio of the pressures in the two vessels.
Use R = 8.31 JK-1 mol-1
2 g of hydrogen is sealed in a vessel of volume 0.02 m3 and is maintained at 300 K. Calculate the pressure in the vessel.
Use R=8.3J K-1 mol-1
Air is pumped into an automobile tyre's tube up to a pressure of 200 kPa in the morning when the air temperature is 20°C. During the day the temperature rises to 40°C and the tube expands by 2%. Calculate the pressure of the air in the tube at this temperature.
Is a slow process always isothermal? Is a quick process always adiabatic?
Two glass bulbs of equal volume are connected by a narrow tube and are filled with a gas at 0°C at a pressure of 76 cm of mercury. One of the bulbs is then placed in melting ice and the other is placed in a water bath maintained at 62°C. What is the new value of the pressure inside the bulbs? The volume of the connecting tube is negligible.
Three samples A, B and C of the same gas (γ = 1.5) have equal volumes and temperatures. The volume of each sample is doubled, the process being isothermal for A, adiabatic for B and isobaric for C. If the final pressures are equal for the three samples, find the ratio of the initial pressures.
A barometer tube is 80 cm long (above the mercury reservoir). It reads 76 cm on a particular day. A small amount of water is introduced in the tube and the reading drops to 75.4 cm. Find the relative humidity in the space above the mercury column if the saturation vapour pressure at the room temperature is 1.0 cm.
The human body has an average temperature of 98°F. Assume that the vapour pressure of the blood in the veins behaves like that of pure water. Find the minimum atmospheric pressure which is necessary to prevent the blood from boiling. Use figure for the vapour pressures.

A faulty barometer contains certain amount of air and saturated water vapour. It reads 74.0 cm when the atmospheric pressure is 76.0 cm of mercury and reads 72.10 cm when the atmospheric pressure is 74.0 cm of mercury. Saturation vapour pressure at the air temperature = 1.0 cm of mercury. Find the length of the barometer tube above the mercury level in the reservoir.
The temperature and humidity of air are 27°C and 50% on a particular day. Calculate the amount of vapour that should be added to 1 cubic metre of air to saturate it. The saturation vapour pressure at 27°C = 3600 Pa.
Use R = 8.3 J K-1 mol-1
A bucket full of water is placed in a room at 15°C with initial relative humidity 40%. The volume of the room is 50 m3. (a) How much water will evaporate? (b) If the room temperature is increased by 5°C, how much more water will evaporate? The saturation vapour pressure of water at 15°C and 20°C are 1.6 kPa and 2.4 kPa respectively.
Use R = 8.3 J K-1 mol-1
If 1022 gas molecules each of mass 10-26 kg collide with a surface (perpendicular to it) elastically per second over an area of 1 m2 with a speed of 104 m/s, the pressure exerted by the gas molecules will be of the order of ______.
Air separated from the atmosphere by a column of mercury of length h = 15 cm is present in a narrow cylindrical two-soldered at one end. When the tube is placed horizontally the air occupies a volume V1 = 240 mm3. When it is set vertically with its open end upwards the volume of the air is V2 = 200 mm3. The atmospheric pressure during the experiment is 7n cm of Hg where n is a single digit number. n will be ______.
