Advertisements
Advertisements
प्रश्न
Calculate the binding energy of an alpha particle given its mass to be 4.00151 u.
उत्तर
Data: M = 4.00151 u, mp = 1.00728 u, mn = 1.00866 u, 1 u = 931.5 MeV/c2
The binding energy of an alpha particle =
(Zmp + Nmn - M)c2
= (2mp + 2mn - M)c2
= [(2)(1.00728 u) + 2(1.00866 u) - 4.00151 u]c2
= (2.01456 + 2.01732 - 4.00151)(931.5) MeV
= 28.289655 MeV
= 28.289655 × 106 eV × 1.602 × 10-10 J
= 4.532002731 × 10-12 J
APPEARS IN
संबंधित प्रश्न
Write symbolically the nuclear β+ decay process of `""_6^11C` Is the decayed product X an isotope or isobar of (`""_6^11C`)? Given the mass values m (`""_6^11C`) = 11.011434 u and m (X) = 11.009305 u. Estimate the Q-value in this process.
Is the nucleus formed in the decay of the nucleus `""_11^22Na`, an isotope or isobar?
Obtain the binding energy (in MeV) of a nitrogen nucleus `(""_7^14"N")`, given `"m"(""_7^14"N")` = 14.00307 u.
The neutron separation energy is defined as the energy required to remove a neutron from the nucleus. Obtain the neutron separation energies of the nuclei `""_20^41"Ca"` and `""_13^27 "Al"` from the following data:
`"m"(""_20^40"Ca")` = 39.962591 u
`"m"(""_20^41"Ca")` = 40.962278 u
`"m"(""_13^26"Al")` = 25.986895 u
`"m"(""_13^27"Al")` = 26.981541 u
What is meant by the terms half-life of a radioactive substance and binding energy of a nucleus?
What is the significance of binding energy per nucleon of a nucleus of a radioactive element?
Define half-life of a radioactive substance
Define the terms (i) half-life (T1/2) and (ii) average life (τ). Find out their relationships with the decay constant (λ).
Use this graph to explain the release of energy in both the processes of nuclear fusion and fission.
What characteristic property of nuclear force explains the constancy of binding energy per nucleon (BE/A) in the range of mass number ‘A’ lying 30 < A < 170?
Which property of nuclear force explains the constancy of binding energy per nucleon `((BE)/A)` for nuclei in the range 20< A < 170 ?
What is the minimum energy which a gamma-ray photon must possess in order to produce electron-positron pair?
Calculate mass defect and binding energy per nucleon of `"_10^20 Ne`, given
Mass of `"_10^20 Ne= 19.992397` u
Mass of `"_0^1H = 1.007825` u
Mass of `"_0^1n = 1.008665` u
In a nuclear reactor, what is the function of:
(i) The moderator
(ii) The control rods
(iii) The coolant
Sketch a graph showing the variation of binding energy per nucleon of a nucleus with its mass number.
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
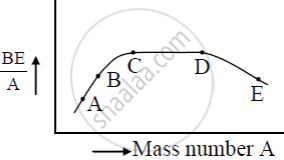
Answer the following question.
Draw the curve showing the variation of binding energy per nucleon with the mass number of nuclei. Using it explains the fusion of nuclei lying on the ascending part and fission of nuclei lying on the descending part of this curve.
An electron in hydrogen atom stays in its second orbit for 10−8 s. How many revolutions will it make around the nucleus at that time?
In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are\[\ce{_12^24Mg}\](23.98504 u), \[\ce{_12^25Mg}\] (24.98584 u), and \[\ce{_12^26Mg}\] (25.98259 u). The natural abundance of \[\ce{_12^24Mg}\] is 78.99% by mass. Calculate the abundances of other two isotopes.
Determine the binding energy per nucleon of the americium isotope \[\ce{_95^244Am}\], given the mass of \[\ce{_95^244Am}\] to be 244.06428 u.
The difference in mass of a nucleus and its constituents is called ______.
A body's centre of mass
Mx and My denote the atomic masses of the parent and the daughter nuclei respectively in a radioactive decay. The Q-value for a β– decay is Q1 and that for a β+ decay is Q2. If m e denotes the mass of an electron, then which of the following statements is correct?
Tritium is an isotope of hydrogen whose nucleus Triton contains 2 neutrons and 1 proton. Free neutrons decay into `p + bare + barν`. If one of the neutrons in Triton decays, it would transform into He3 nucleus. This does not happen. This is because ______.
Heavy stable nucle have more neutrons than protons. This is because of the fact that ______.
The deuteron is bound by nuclear forces just as H-atom is made up of p and e bound by electrostatic forces. If we consider the force between neutron and proton in deuteron as given in the form of a Coulomb potential but with an effective charge e′: F = `1/(4πε_0) e^('2)/r` estimate the value of (e’/e) given that the binding energy of a deuteron is 2.2 MeV.
Nuclei with magic no. of proton Z = 2, 8, 20, 28, 50, 52 and magic no. of neutrons N = 2, 8, 20, 28, 50, 82 and 126 are found to be very stable.
(i) Verify this by calculating the proton separation energy Sp for 120Sn (Z = 50) and 121Sb = (Z = 51).
The proton separation energy for a nuclide is the minimum energy required to separate the least tightly bound proton from a nucleus of that nuclide. It is given by `S_P = (M_(z-1^' N) + M_H - M_(ZN))c^2`.
Given 119In = 118.9058u, 120Sn = 119.902199u, 121Sb = 120.903824u, 1H = 1.0078252u.
(ii) What does the existance of magic number indicate?
Explain the release of energy in nuclear fission and fusion on the basis of binding energy per nucleon curve.
Calculate the binding energy of an alpha particle in MeV. Given
mass of a proton = 1.007825 u
mass of a neutron = 1.008665 u
mass of He nucleus = 4.002800 u
1u = 931 MeV/c2
Define binding energy per nucleon.
State the significance of binding energy per nucleon.
Which of the following quantities is a measure of stability of nucleus?
What is meant by “binding energy per nucleon” of a nucleus?
