Advertisements
Advertisements
प्रश्न
State any two uses of diamond.
उत्तर
Uses of diamonds:
(1) As diamonds have an extraordinary shine, they are used in making jewellery.
(2) Diamonds, due to their hardness, are used in instruments like glass cutters, grinders. They are also used as abrasives for polishing.
APPEARS IN
संबंधित प्रश्न
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – the eight atoms of sulphur are joined together in the form of a ring.)
Butanone is a four-carbon compound with the functional group:
Name the following:
The property of element by virtue of which atoms of the element can link to each other in the form of a long chain or ring structure.
Friedrich Wohler converted an inorganic compound into an organic compound in the laboratory.
Write the name and formula of organic compound formed.
Draw the electron-dot structure of CO2 compound and state the type of bonding.
Draw the electron-dot structure of H2O compound and state the type of bonding.
Draw the electron-dot structure of NH3 and state the type of bonding.
A covalent molecule having a double bond between its atoms is:
(a) Hydrogen
(b) Oxygen
(c) water
(d) ammonia
For each compound mentioned above give the formulae of ions formed in aqueous solution.
The following structural formula belongs to which carbon compound?
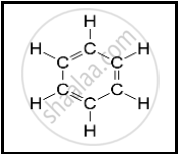
The carbon compound is used in daily life is ______.
Explain the structure of Ammonium ion.
Explain why Carbon tetrachloride does not dissolve in water.
What are the term defined below?
A bond formed by share pair of electrons, each bonding atom contributing one electron to the pair.
Draw the electron dot diagram and structure of :
methane
Name the anion present in the following compound:
When a barium chloride solution is added to a solution of compound B, a white precipitate insoluble in dilute hydrochloric acid is formed.
Considering MgCl2 as ionic compound and CH4 as covalent compound give any two differences between these two compounds.
Which of the following is the purest form of carbon?
The table shows the electronic structures of four elements.
| Element | Electronic Structure |
| P | 2, 6 |
| Q | 2, 8, 1 |
| R | 2, 8, 7 |
| S | 2, 8, 8 |
- Identify which element(s) will form covalent bonds with carbon.
- “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
Explain dipole (polar) molecule by taking hydrogen chloride as an example.
