Advertisements
Advertisements
प्रश्न
Describe one reaction of a carboxylic acid.
उत्तर
Ethanoic acid is a carboxylic acid which when reacts with ethanol (alcohol) produces sweet smelling ester. The process in which the carboxylic acid reacts with alcohol, in the presence of a catalyst, to form an ester is called esterification.
The chemical equation for the above reaction is as follows:
`CH_3 COOH +_2 H_5 OH` 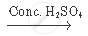 `CH_3 COOC_2 H_5 + H_2O`
`CH_3 COOC_2 H_5 + H_2O`
APPEARS IN
संबंधित प्रश्न
A student adds a spoon full of powdered sodium hydrogen carbonate to a flask containing ethanoic acid. List two main observations, he must note in his note book, about the reaction that takes place. Also write chemical equation foe the reaction.
You have four test tubes, A, B, C and D containing sodium carbonate, sodium chloride, lime water and blue litmus solutions respectively. Out of these the material of which test tube/ test tubes would be suitable for the correct test of acetic/ethanoic acid?
(a) only A
(b) A and B
(c) B and C
(d) A and D
Consider the following organic compounds:
HCHO, C2H5OH, C2H6, CH3COOH, C2H5CI
Choose two compounds which can react in the presence of conc. H2SO4 to form an ester. Give the name and formula of the ester formed.
Acetic acid is a typical acid. Write one equation in case of its reactions with a carbonate.
If you are asked to report your observations about the following two properties of acetic acid, what would you report?
(i) Odour
(ii) Effect on litmus
Explain the following term with an example.
Oxidant
Bubbles are seen in the test tube during the preparation of lime water.
A spatula full of sodium carbonate is taken in a test tube and 2 mL of dilute ethanoic acid is added to it.
Write a chemical equation for the reaction.
Write the chemical equation for the ethanol to ethanoic acid of an oxidation reaction.
Give the balanced chemical equation of the reaction.
Oxidation of ethanol by acidified potassium dichromate.
