Advertisements
Advertisements
प्रश्न
Draw the simple Fisher projection formulae of D - (+) - glucose and D - (-) - fructose
उत्तर


APPEARS IN
संबंधित प्रश्न
Write the reaction that indicates the presence of -CHO group in glucose
What happens when glucose is treated with hydrogen cyanide?
Write the product when D-glucose reacts with conc. HNO3.
What do you observe when glucose solution is heated with Tollen’s reagent?
Write the reactions involved when D-glucose is treated with the following reagent:
H2N-OH
The following compound can be called as:
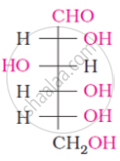
The number of asymmetric carbon atom(s) below the figure is/are

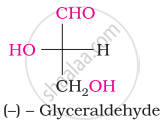
Oxime is formed by treating glucose with ____________.
Glucose does not give Schiff’s test because of the formation of cyclic ____________.
Which one of the following compounds is different from the rest?
Glucose reacts with acetic anhydride to form ______.
Which of the following properties of glucose cannot be explained by its open chain structure?
(i) Glucose does not form hydrogen sulphite with NaHSO3.
(ii) On oxidation with HNO3 glucose gives saccharic acid.
(iii) Glucose is found to exist in two different crystalline forms which are named as α and β.
Glucose is found to exist in two different α and β crystalline forms. These forms can be obtained by:
(i) The α form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(ii) The β form of glucose is obtained by crystallisation from a concentrated solution of glucose at 303 K.
(iii) The β form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
(iv) The α form is obtained by crystallisation from hot and saturated aqueous solution at 371 K.
Reduction of glucose by HI suggest that ____________.
Which of the following reactions of glucose can be explained only by its cyclic structure?
Which one is correct?
Which one of the following reactions is not explained by the open chain Structure of glucose?
In the following reaction, identify A and B:
\[\begin{array}{cc}
\ce{C6H12O6 ->[Acetic anhydride] A}\\
\downarrow \text{Conc. nitric acid}\phantom{...}\\
\ce{B}\phantom{.................}\end{array}\]
Why does compound (A) given below not form an oxime?
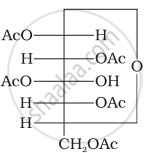
(A)
How will you distinguish 1° and 2° hydroxyl groups present in glucose? Explain with reactions.
Assertion: D (+) – Glucose is dextrorotatory in nature.
Reason: ‘D’ represents its dextrorotatory nature.
Write the reactions of D-glucose which can’t be explained by its open-chain structure. How can cyclic structure of glucose explain these reactions?
On the basis of which evidences D-glucose was assigned the following structure?
\[\begin{array}{cc}
\ce{CHO}\\
|\phantom{....}\\
\phantom{..}\ce{(CHOH)4}\\
|\phantom{....}\\
\phantom{..}\ce{CH2OH}
\end{array}\]
Account for the following:
There are 5 OH groups in glucose
Account for the following:
What happens when D – glucose is treated with the following reagents
Bromine water
Account for the following:
What happens when D – glucose is treated with the following reagents
HNO3
Consider the following reactions:
(i) \[\ce{Glucose + R-OH ->[Conc. HNO3] [A] ->[X eq of][(CH3CO)2O] Acetyl derivative}\]
(ii) \[\ce{Glucose ->[Ni/H2] [A] ->[Y eq of][(CH3CO)2O] Acetyl derivative}\]
(iii) \[\ce{Glucose ->[Z eq of][(CH3CO)2O] Acetyl derivative}\]
'X, 'Y' and 'Z' in these reactions are respectively:
