Advertisements
Advertisements
प्रश्न
Draw structure and name the shape of bromine trifluoride.
उत्तर
Structure of bromine trifluoride:
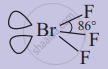
Shape of bromine trifluoride: Bent T-shaped
APPEARS IN
संबंधित प्रश्न
Draw the structures of the following molecules: XeF4
Account for the following:
Helium is used in diving apparatus.
Which noble gas has the lowest boiling point?
Write the structures of the following molecules: XeOF4
Complete the following equations : XeF4 + O2F2→
Complete the following equation:
XeF2 + H2O →
test q 1234
Balance the following equation: XeF6 + H2O → XeO2F2 + HF
Why has it been difficult to study the chemistry of radon?
What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Which one of the following does not exist?
(i) XeOF4
(ii) NeF2
(iii) XeF2
(iv) XeF6
Give the formula and describe the structure of a noble gas species which is isostructural with
`ICI_4^(-)`
Give the formula and describe the structure of a noble gas species which is isostructural with:
`IBr_2^(-)`
Give the formula and describe the structure of a noble gas species which is isostructural with:
`BrO_3^(-)`
Answer the following.
List the uses of Neon and argon gases.
Write electronic configuration and two uses of neon. (Z = 10)
Complete the given chemical equations:
`2XeF_2 + 2H_2O->`
Complete the following reactions:
XeF6+2H2O ----->
Draw the structures of the following
XeF6
Write the electronic configuration of the following element:
Krypton (Z = 36)
Fill in the blanks by choosing the appropriate word/words from the brackets
(square pyramidal, electrical, 74; 26, sp3d2, sp3d, chemical, 68, 32, tetrahedral, yellow, white, iodoform, Lucas)
The geometry of XeOF4 molecule is ______ and the hybridisation of Xenon atom in the molecule is ________.
Draw the structure of XeF4.
Sulfur dioxide reacts with sodium hydroxide solution to form _______.
The number of lone pairs of electrons present in ClF5:
Explain the trend in the following atomic properties of group 16 elements:
Electron gain enthalpy
Which of the following fluorides does not exist?
Partial hydrolysis of XeF4 gives ____________.
Which of the following reactions is an example of redox reaction?
Substance having the lowest boiling point ______.
Match List - I with List - II:
| List - I | List - II | ||
| (Species) | (Number of lone pairs of electrons on the central atom) |
||
| (A) | XeF2 | (i) | 0 |
| (B) | XeO2F2 | (ii) | 1 |
| (C) | XeO3F2 | (iii) | 2 |
| (D) | XeF4 | (iv) | 3 |
Choose the most appropriate answer from the options given below:
Which one of the following reactions of xenon compounds is not feasible?
\[\ce{XeF6 + H2O ->[Partial][Hydrolysis] \underline{}\underline{}\underline{}\underline{} + \underline{}\underline{}\underline{}\underline{}}\]
Write two uses of neon.
