Advertisements
Advertisements
प्रश्न
Give the formula and describe the structure of a noble gas species which is isostructural with:
`IBr_2^(-)`
उत्तर १
XeF2 is isoelectronic to `IBr_2^(-)` and has a linear structure.
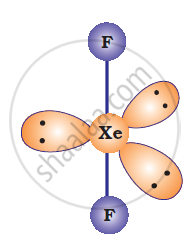
उत्तर २
IBr2–: In IBr2–, central atom I has eight electrons. Two of these are utilized in forming two single bonds with two Br atom. Six remaining electrons constitutes three lone pairs. It is arranged in linear structure.

IBr2– has 22 valence electrons. A noble gas species having 22 valence electrons is XeF2 (8+2 x 7=22).
XeF2 is also linear.
APPEARS IN
संबंधित प्रश्न
Account for the following:
Helium is used in diving apparatus.
Which noble gas has the lowest boiling point?
Complete the following equations : XeF4 + O2F2→
Draw the structures of the following:
XeO3
Draw the structures of the following
XeF6
Draw the structure of XeF4.
Sulfur dioxide reacts with sodium hydroxide solution to form _______.
Draw structure and name the shape of bromine trifluoride.
Explain the trend in the following atomic properties of group 16 elements:
Electron gain enthalpy
Which of the following fluorides does not exist?
Match the compounds given in Column I with the hybridisation and shape given in Column II and mark the correct option.
| Column I | Column II |
| (A) XeF6 | (1) sp3d3 – distorted octahedral |
| (B) XeO3 | (2) sp3d2 – square planar |
| (C) XeOF4 | (3) sp3 – pyramidal |
| (D) XeF4 | (4) sp3d2 – square pyramidal |
On partial hydrolysis, XeF6 gives ______.
The atomic radii of Zr and Hf are almost identical. This is because of
The order of increasing sizes of atomic radii among the elements O, S, Se and As is:
Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
Which one of the following reactions of xenon compounds is not feasible?
Write two uses of neon.
