Advertisements
Advertisements
प्रश्न
How much heat energy is necessary to raise the temperature of 5 kg of water from 20°C to 100°C?
उत्तर
Given: Mass (m) = 5 kg, specific heat of water (c) = 1 kcal/kg °C
Change in temperature (ΔT) = 100 − 20 = 80°C
To find: Heat energy (Q)
Formula: Q = m c ΔT
Calculation:
According to the principle of heat exchange,
Energy supplied to water = Energy gained by water
From the formula,
Q = 5 × 1 × 80 = 400 kcal
Heat energy necessary to raise the temperature of the water is 400 kcal.
APPEARS IN
संबंधित प्रश्न
Water in lakes and ponds do not freeze at once in cold countries. Give a reason is support of your answer.
During the phase change does the average kinetic energy of the molecules of the substance increase?
State S.I. unit of specific heat capacity.
Discuss the role of high specific heat capacity of water with reference to climate in coastal areas.
Name three fossil fuels that emit carbon dioxide into the atmosphere ?
Which principle is used to measure the specific heat capacity of a substance?
A calorimeter has mass 100 g and specific heat 0.1 kcal/ kg °C. It contains 250 gm of liquid at 30°C having specific heat of 0.4 kcal/kg °C. If we drop a piece of ice of mass 10 g at 0°C, What will be the temperature of the mixture?
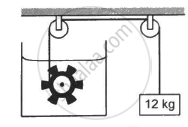
Figure shows a paddle wheel coupled to a mass of 12 kg through fixed frictionless pulleys. The paddle is immersed in a liquid of heat capacity 4200 J K−1 kept in an adiabatic container. Consider a time interval in which the 12 kg block falls slowly through 70 cm. (a) How much heat is given to the liquid? (b) How much work is done on the liquid? (c) Calculate the rise in the temperature of the liquid neglecting the heat capacity of the container and the paddle.
The substances like water which have ........... Heat capacity warm up more slowly than substances like iron which have .......... heat capacity.
What are the factors on which the quantity of heat given to a body depends?
The farmers fill their fields with water in winter. Give reason.
Explain, Why is it advisabile to pour cold water over burns, caused on human body, by hot solids?
Solve the following problem.
Specific latent heat of vaporization of water is 2.26 × 106 J/kg. Calculate the energy needed to change 5.0 g of water into steam at 100 ºC.
State factors on which the amount of heat radiated by a body depends.
Read this activity and answer the following questions.
- Take three spheres of iron, copper and lead. the lead of equal mass.
- Put all the three spheres in boiling water in the beaker for some time.
- Take the three spheres out of the water.
- All the spheres will be at a temperature 100 °C.
- Put them immediately on the thick slab of wax.
- Note, the depth that each of the sphere goes into the wax.
Questions:
- Which property is determined from this activity?
- Give name to that property.
- Explain the term principal of heat exchange with the help of this activity.
What is specific heat capacity?
Two uniform brass rods A and B of length land 2l and radii 2r and r respectively are heated to the same temperature. The ratio of the increase in the volume ofB to that of A is ____________.
The molar specific heats of an ideal gas at constant pressure and constant volume are denoted by Cp and Cv respectively. If `gamma = "C"_"p"/"C"_"v"` and R is the universal gas constant, then Cp is equal to ______.
A metal ball of heat capacity 50J/°C loses 2000 J of heat. By how much will its temperature fall?
